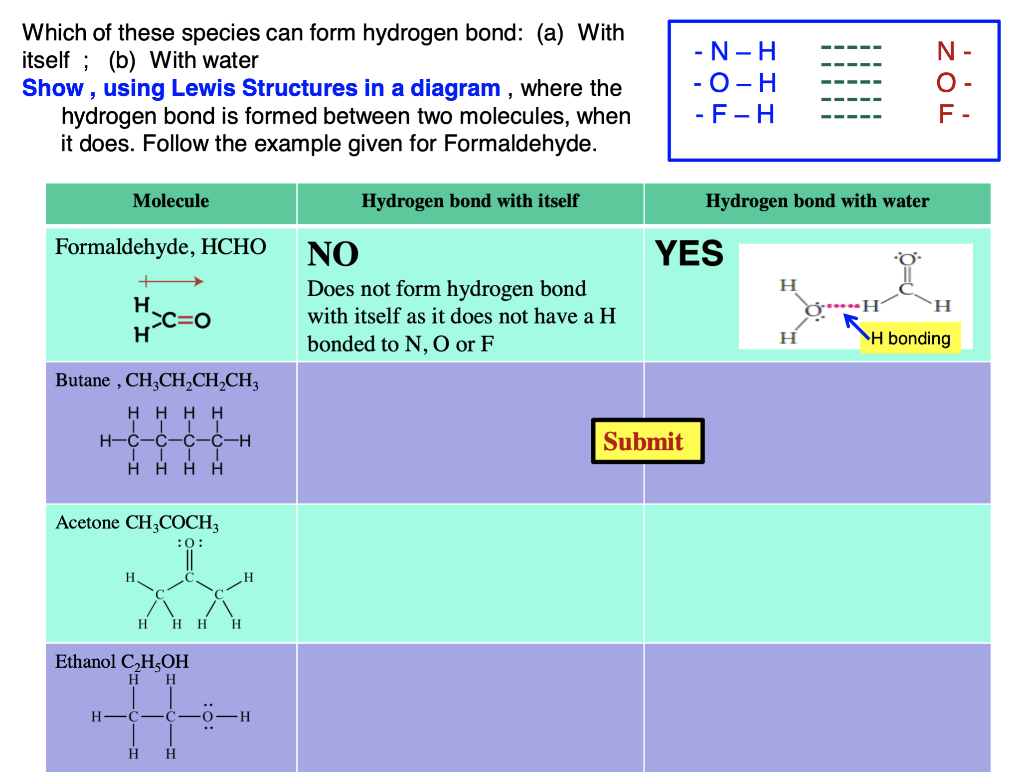
Can Acetone Form Hydrogen Bonds
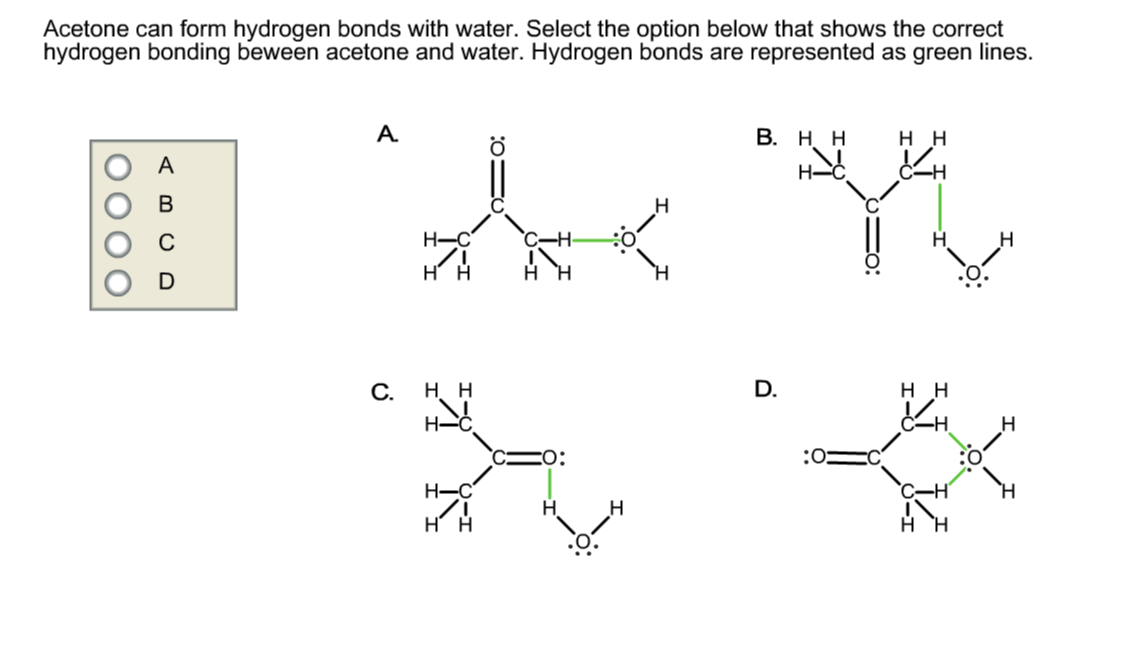
Can Acetone Form Hydrogen Bonds - Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water.
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone.
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water.
SOLVED2. Can a molecule of acetone form a hydrogen bond with water
Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water.
Answered Can Butane, Acetone, and Ethanol form a… bartleby
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been.
Acetone Lewis Structure With Polarity
Acetone can form hydrogen bonds with water. Four images are given which show an acetone molecule interacting with a water. The water molecule forms two hydrogen bonds with acetone: The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone.
SOLVEDWhy can't two molecules of acetone form a hydrogen bond with
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Four images are given which show an acetone molecule interacting with a water. Acetone can form hydrogen bonds with water. The water molecule forms.
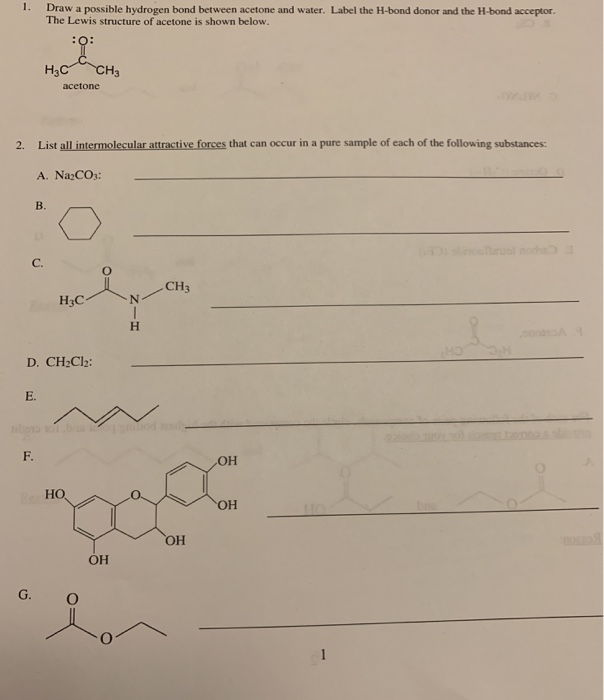
Solved 1. Draw a possible hydrogen bond between acetone and
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Four images are given which show an acetone molecule interacting with a water. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the.
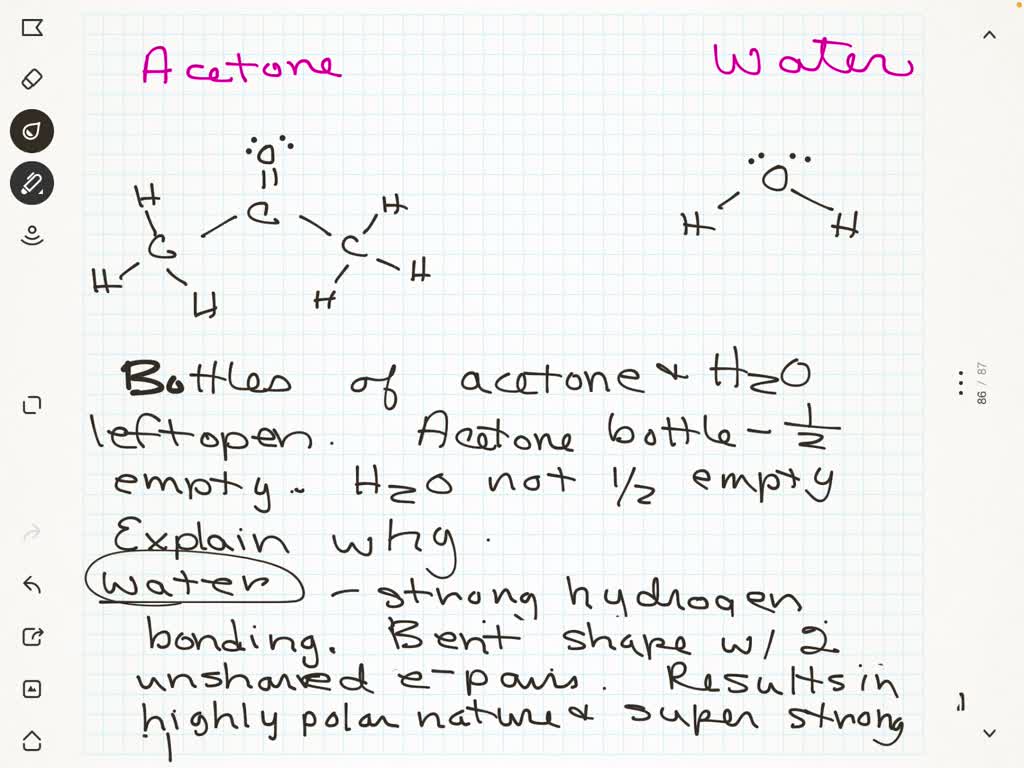
I had to draw Hbonds between water and Acetone. Did I do it correctly
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. The water molecule forms two hydrogen bonds with acetone: Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming..
hydrogen bond acetone ethanol YouTube
Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms.
SOLVED How many hydrogen bonds can form between an acetone molecule
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the.
SOLVED SOURCE CHAPTER PROBLEM TOPIC INTRAMOLECULAR FORCES The
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone does not have a hydrogen atom.
Solved Acetone can form hydrogen bonds with water. Select
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. The water molecule forms two hydrogen bonds with acetone: Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone..
Acetone Can Form Hydrogen Bonds With Water.
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. The water molecule forms two hydrogen bonds with acetone: I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone.