Clinical Data Management
Clinical Data Management - It aims to ensure data quality , integrity, and compliance with internal. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. Clinical data management (cdm) is a set of practices that handle information generated during medical research. This course by vanderbilt on coursera focuses on best practices in data.
This course by vanderbilt on coursera focuses on best practices in data. It aims to ensure data quality , integrity, and compliance with internal. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management (cdm) is a set of practices that handle information generated during medical research.
It aims to ensure data quality , integrity, and compliance with internal. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management (cdm) is a set of practices that handle information generated during medical research. This course by vanderbilt on coursera focuses on best practices in data.
Data Management in Clinical Trials JLI Blog
This course by vanderbilt on coursera focuses on best practices in data. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management (cdm) is a set of practices that handle.
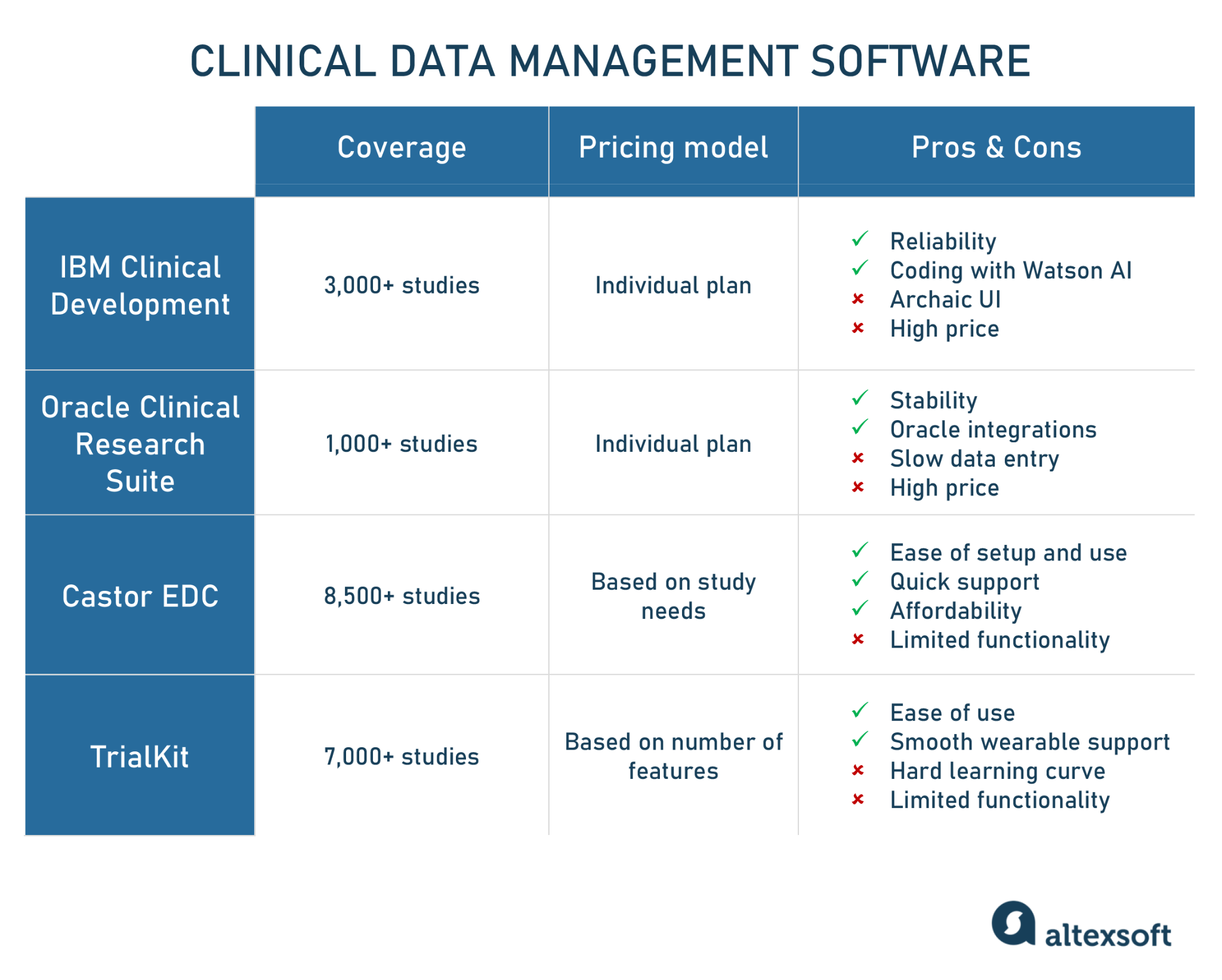
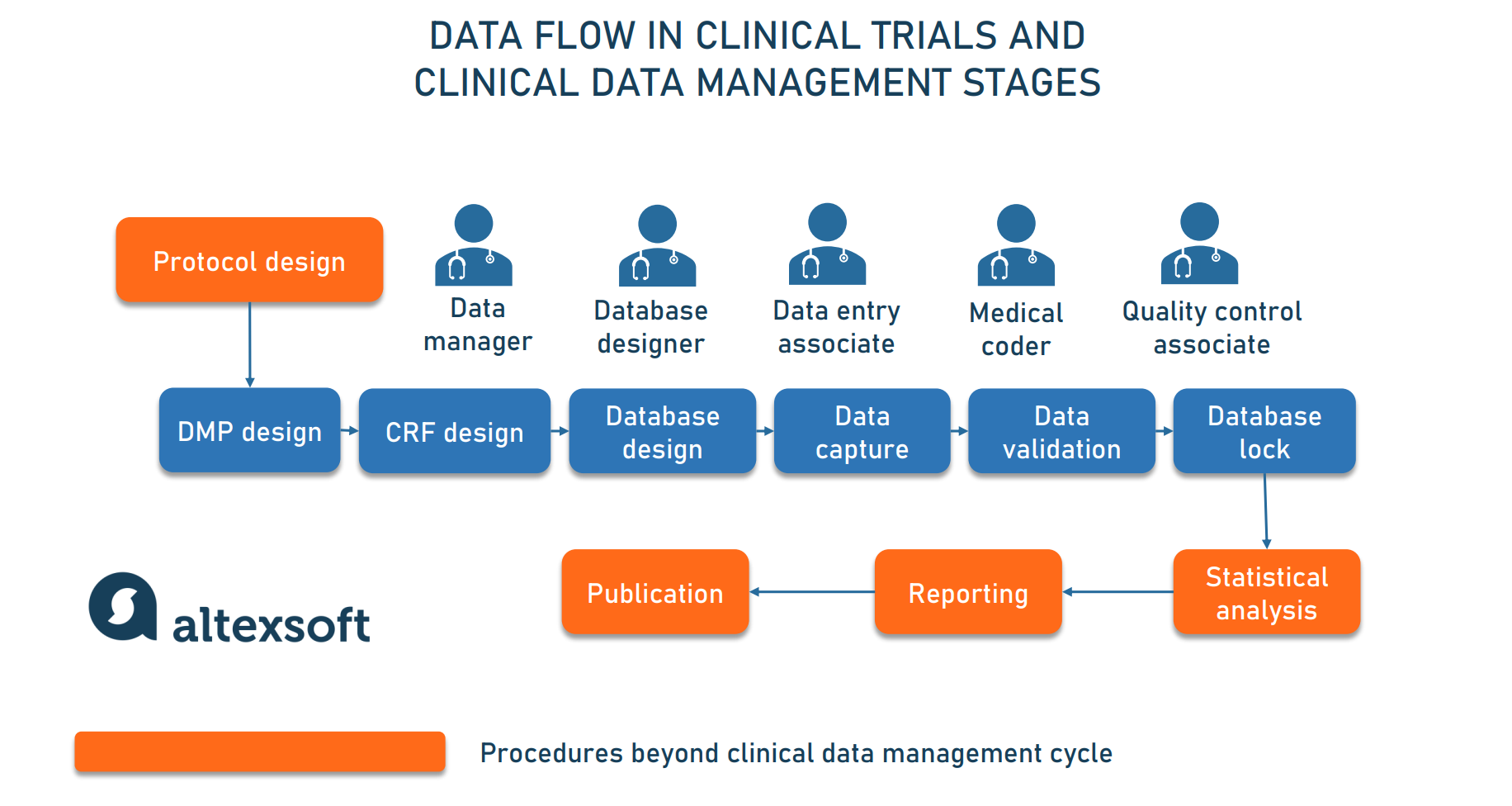
Clinical Data Management Roles, Steps, and Software Tools AltexSoft
Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. It aims to ensure data quality , integrity, and compliance with internal. Clinical data management (cdm) is a set of practices that handle information generated during medical research. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials.
what is Clinical data management by Smita Kathe Issuu
It aims to ensure data quality , integrity, and compliance with internal. Clinical data management (cdm) is a set of practices that handle information generated during medical research. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. This course by vanderbilt on coursera focuses on best practices in.
Clinnovo News Clinical Data Management Process
Clinical data management (cdm) is a set of practices that handle information generated during medical research. This course by vanderbilt on coursera focuses on best practices in data. It aims to ensure data quality , integrity, and compliance with internal. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management is.
The Future of Clinical Trial Data Management
Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. It aims to ensure data quality , integrity, and compliance with internal. This course by vanderbilt on coursera focuses on best practices in data. Clinical data management (cdm) is a set of practices that handle information generated during medical research. Clinical data management is.
Clinical Data Management QualityMedDev
Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. It aims to ensure data quality , integrity, and compliance with internal. This course by vanderbilt on coursera focuses on best practices in.
Clinical Data Management Course UCSC Silicon Valley Extension
Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. This course by vanderbilt on coursera focuses on best practices in data. It aims to ensure data quality , integrity, and compliance with internal. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and.
Clinical Data Management Roles, Steps, and Software Tools AltexSoft
Clinical data management (cdm) is a set of practices that handle information generated during medical research. It aims to ensure data quality , integrity, and compliance with internal. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. This course by vanderbilt on coursera focuses on best practices in.
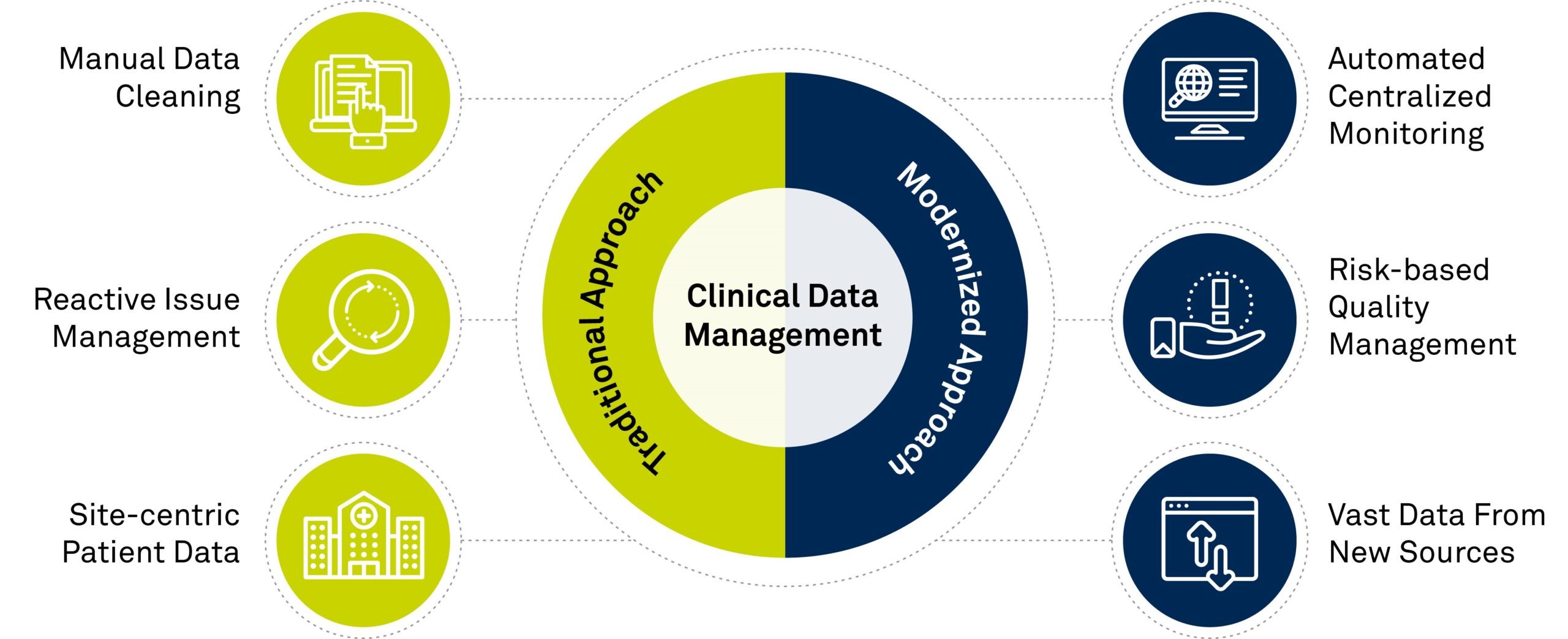
Top 4 Trends In Clinical Data Management
It aims to ensure data quality , integrity, and compliance with internal. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. This course by vanderbilt on coursera focuses on best practices in data. Clinical data management (cdm) is a set of practices that handle information generated during medical research. Clinical data management is.
Clinical Data Management by WorkSure Clinic, Medical coding, Clinical
This course by vanderbilt on coursera focuses on best practices in data. Clinical data management (cdm) is a set of practices that handle information generated during medical research. Learn critical concepts and practical methods for planning, collecting, storing, and disseminating data in clinical research. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials.
Learn Critical Concepts And Practical Methods For Planning, Collecting, Storing, And Disseminating Data In Clinical Research.
Clinical data management (cdm) is a set of practices that handle information generated during medical research. It aims to ensure data quality , integrity, and compliance with internal. Clinical data management is the systematic collection, organization and validation of data obtained from clinical trials to ensure accuracy and reliability. This course by vanderbilt on coursera focuses on best practices in data.