Do All B Group Elements Form More Than One Charge
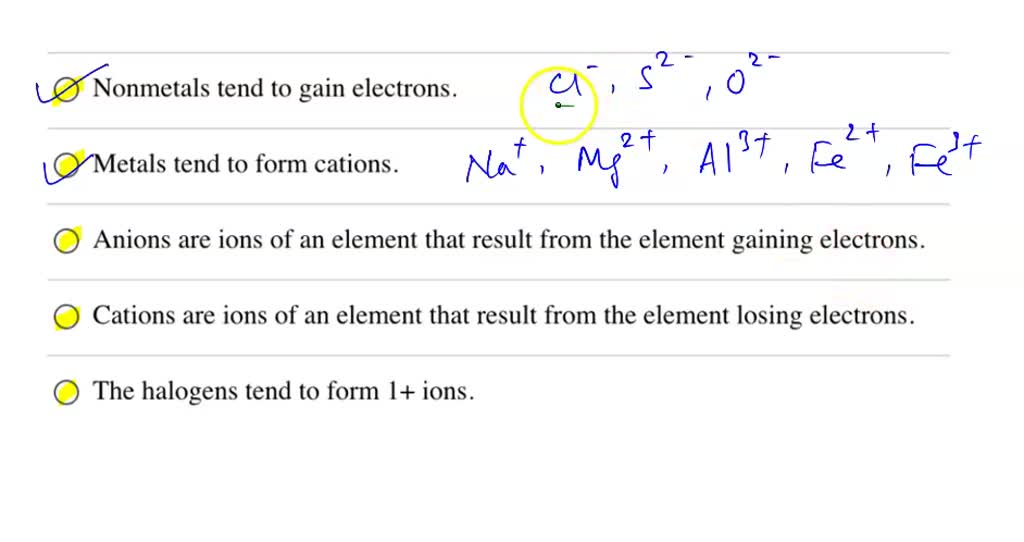
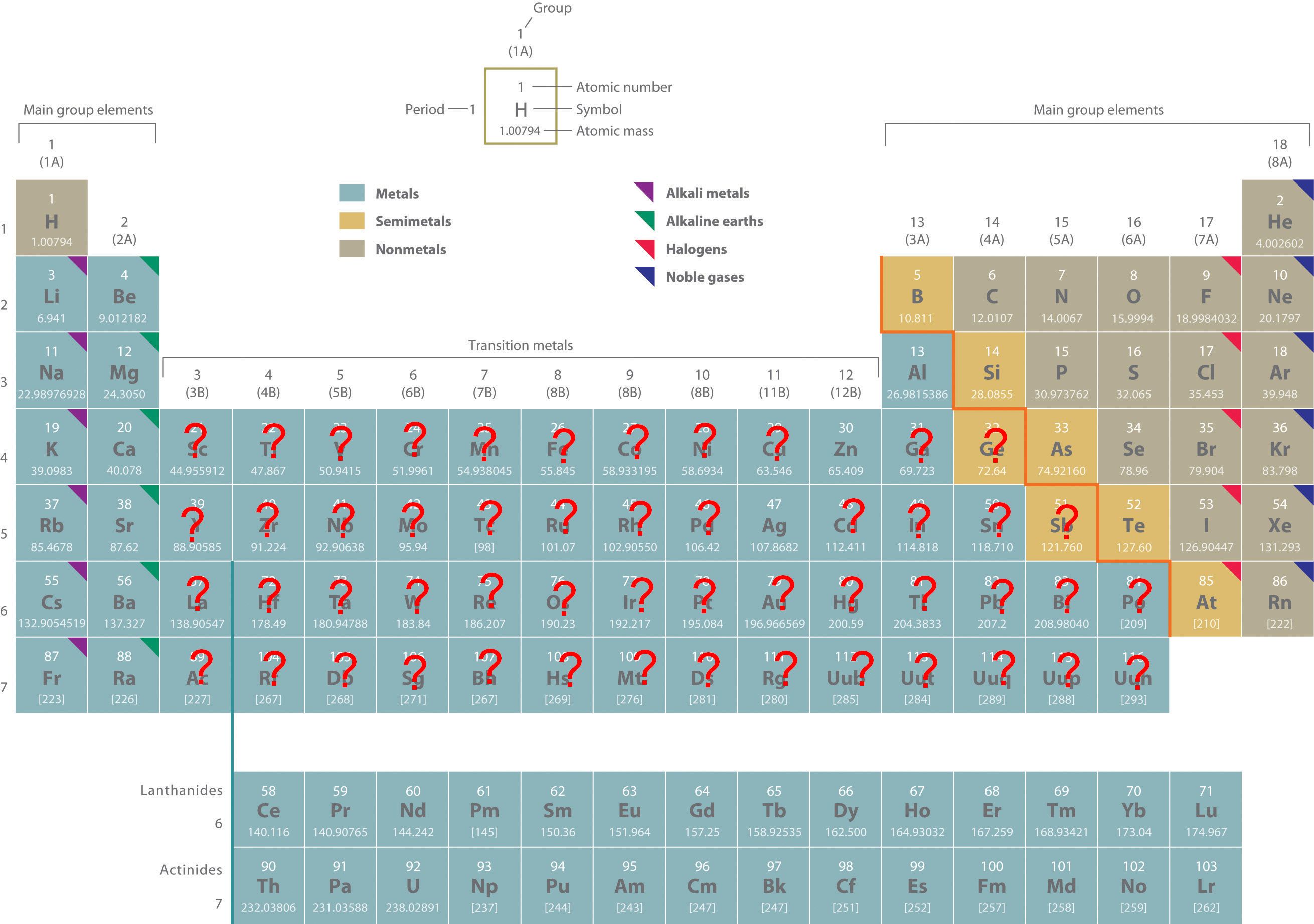
Do All B Group Elements Form More Than One Charge - All b group elements form more than one charge. All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. Study with quizlet and memorize.
All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge. All group 2 elements (alkaline earth metals) lose two. Study with quizlet and memorize.
All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge. All group 2 elements (alkaline earth metals) lose two. Study with quizlet and memorize. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide?
Properties of Elements Biology for NonMajors I
All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge. Study with quizlet and memorize.
How Do Chemists Organize Information About Elements DeangelohasAtkinson
Study with quizlet and memorize. All b group elements form more than one charge. All group 2 elements (alkaline earth metals) lose two. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide?
Naming Ionic Compounds
All group 2 elements (alkaline earth metals) lose two. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. Study with quizlet and memorize. All b group elements form more than one charge. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide?
Main Group Elements Definition
All b group elements form more than one charge. Study with quizlet and memorize. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide?
What Are The Transition Metals On The Periodic Table
All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. Study with quizlet and memorize. All b group elements form more than one charge. All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide?
SOLVED Examining your labeled periodic table, which of the following
All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge. All group 2 elements (alkaline earth metals) lose two. Study with quizlet and memorize. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide?
Zinc group element chemistry Britannica
Study with quizlet and memorize. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge.
Periodic Table With Ionic Charges Labeled Elcho Table
Study with quizlet and memorize. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? All b group elements form more than one charge. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All group 2 elements (alkaline earth metals) lose two.
Element Charges Chart How to Know the Charge of an Atom
All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? Study with quizlet and memorize.
metals tend to form what kind of ions Lombardi Bothe1936
All b group elements form more than one charge. All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. All group 2 elements (alkaline earth metals) lose two. All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? Study with quizlet and memorize.
All Group 2 Elements (Alkaline Earth Metals) Lose Two.
All b group elements form more than one charge, what is the correct formula for vanadium(v) sulfide? All group 1 elements (alkali metals) lose one electron to form an ion with a 1+ charge b. Study with quizlet and memorize. All b group elements form more than one charge.
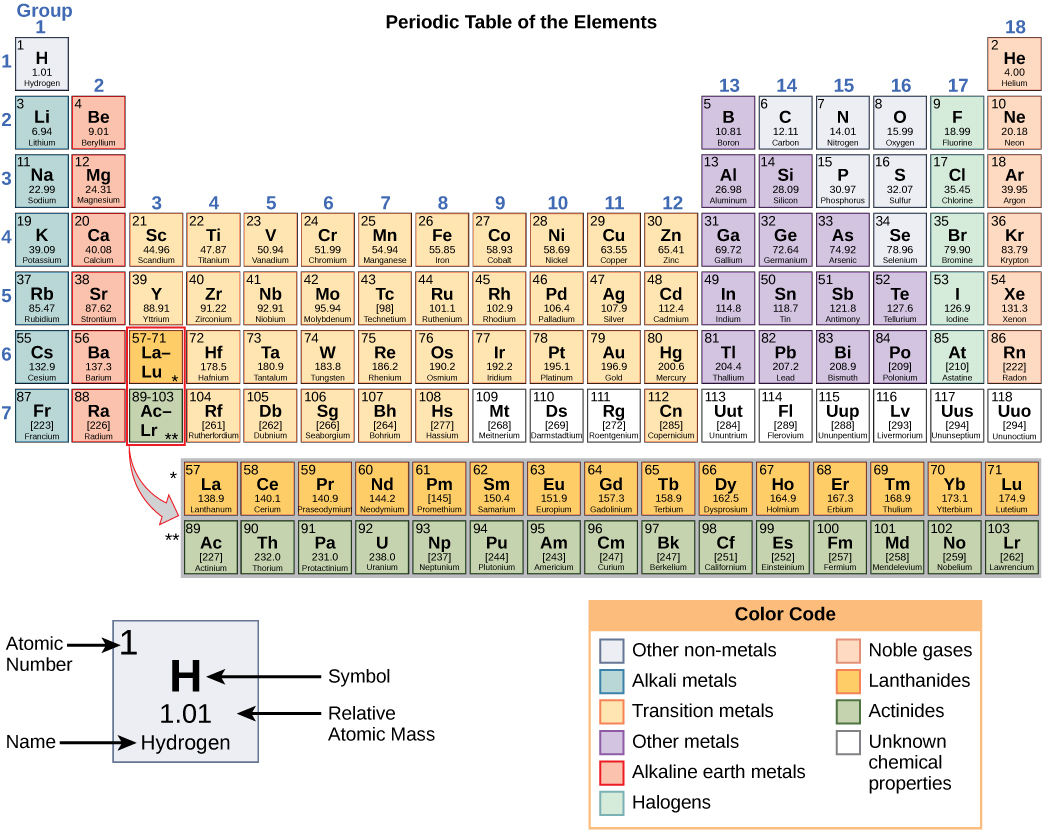


/the-periodic-table--digital-illustration--73016803-598b218ec41244001024af78.jpg)