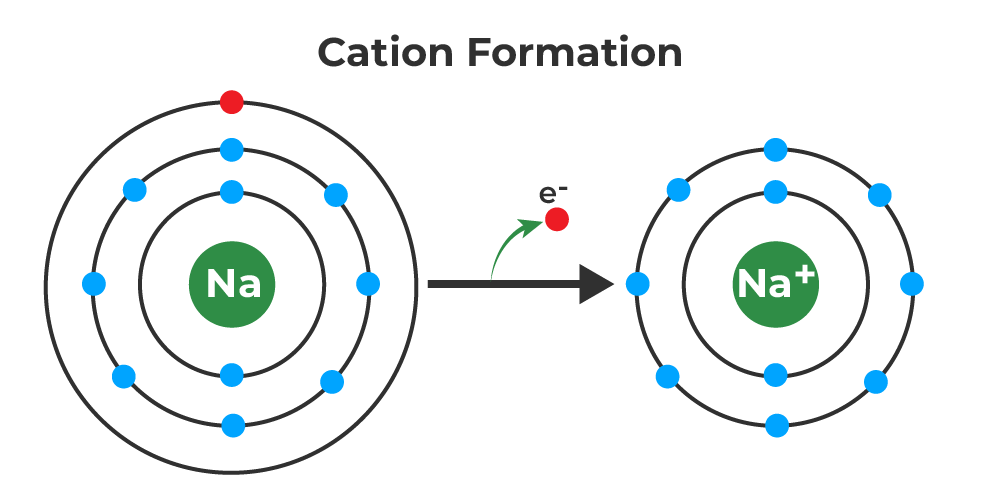
Do Metals Form Cations
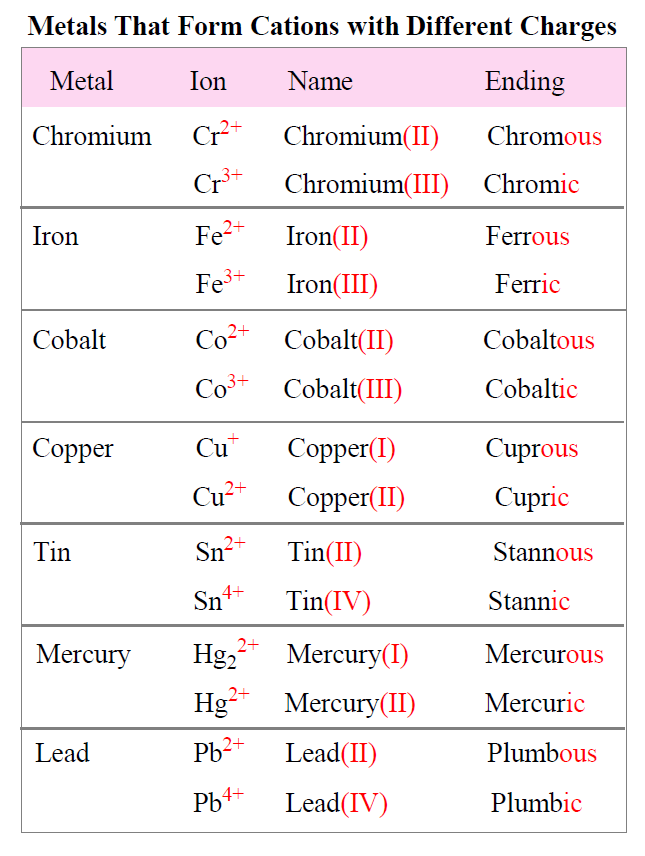
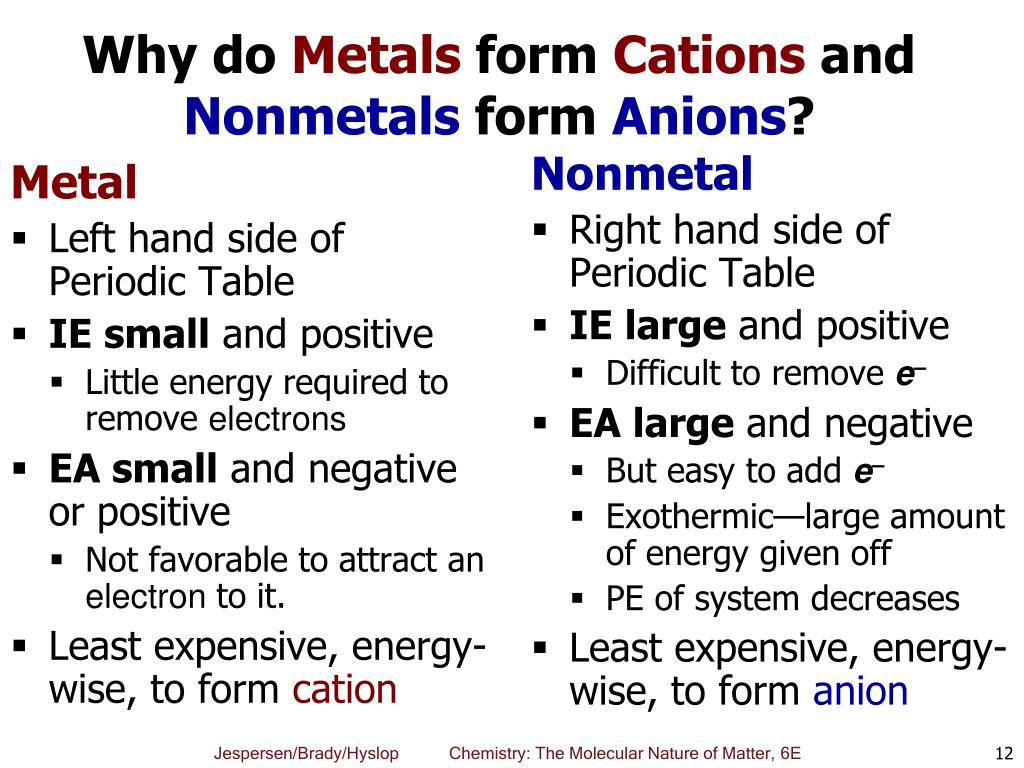
Do Metals Form Cations - Virtually all of the transition metals form. Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Ionization energy is the energy required to remove. Virtually all of the transition metals form. Metals tend to form cations because they have low ionization energy and low electronegativity.
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they have low ionization energy and low electronegativity. Virtually all of the transition metals form. Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge.
PPT Notes for Oct 23 and Oct 24 PowerPoint Presentation, free
Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. Virtually all of the transition metals form. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Ionization.
H2 cation and anion Kangen Water Singapore HydrogenRich Water
Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Virtually all of the transition metals form. Ionization.
Cations vs Anions Difference Between Cations and Anions with Examples
Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals..
Pin on What is an ion?
Virtually all of the transition metals form. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they have low ionization energy and low electronegativity. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Ionization.
Diferencia entre aniónes y catiónes (con nomenclatura y ejemplos
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. Ionization energy is the energy required to remove..
Naming Monatomic and Polyatomic Ions Chemistry Steps
Metals tend to form cations because they have low ionization energy and low electronegativity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Virtually all of the transition metals form. Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Ionization energy is the energy required to remove. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Virtually all of the transition metals form. Metals tend to form cations because they have low ionization energy and low electronegativity. Most transition metals form multiple cations, that is, they have more.
Ionic Compounds Stone Cold Chemistry Talk
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they have low ionization energy and low electronegativity. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Virtually all of the transition metals form. Ionization.
Do Metals Form Anions Or Cations
Metals tend to form cations because they have low ionization energy and low electronegativity. Virtually all of the transition metals form. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Ionization energy is the energy required to remove. With the exception of hydrogen, all elements that form positive ions by losing.
Do Metals Form Anions Or Cations
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Virtually all of the transition metals form. Metals tend to form cations because they have low ionization energy and low electronegativity. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Ionization.
With The Exception Of Hydrogen, All Elements That Form Positive Ions By Losing Electrons During Chemical Reactions Are Called Metals.
Virtually all of the transition metals form. Ionization energy is the energy required to remove. Metals tend to form cations because they have low ionization energy and low electronegativity. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge.