Enthalpy Of Lattice Formation
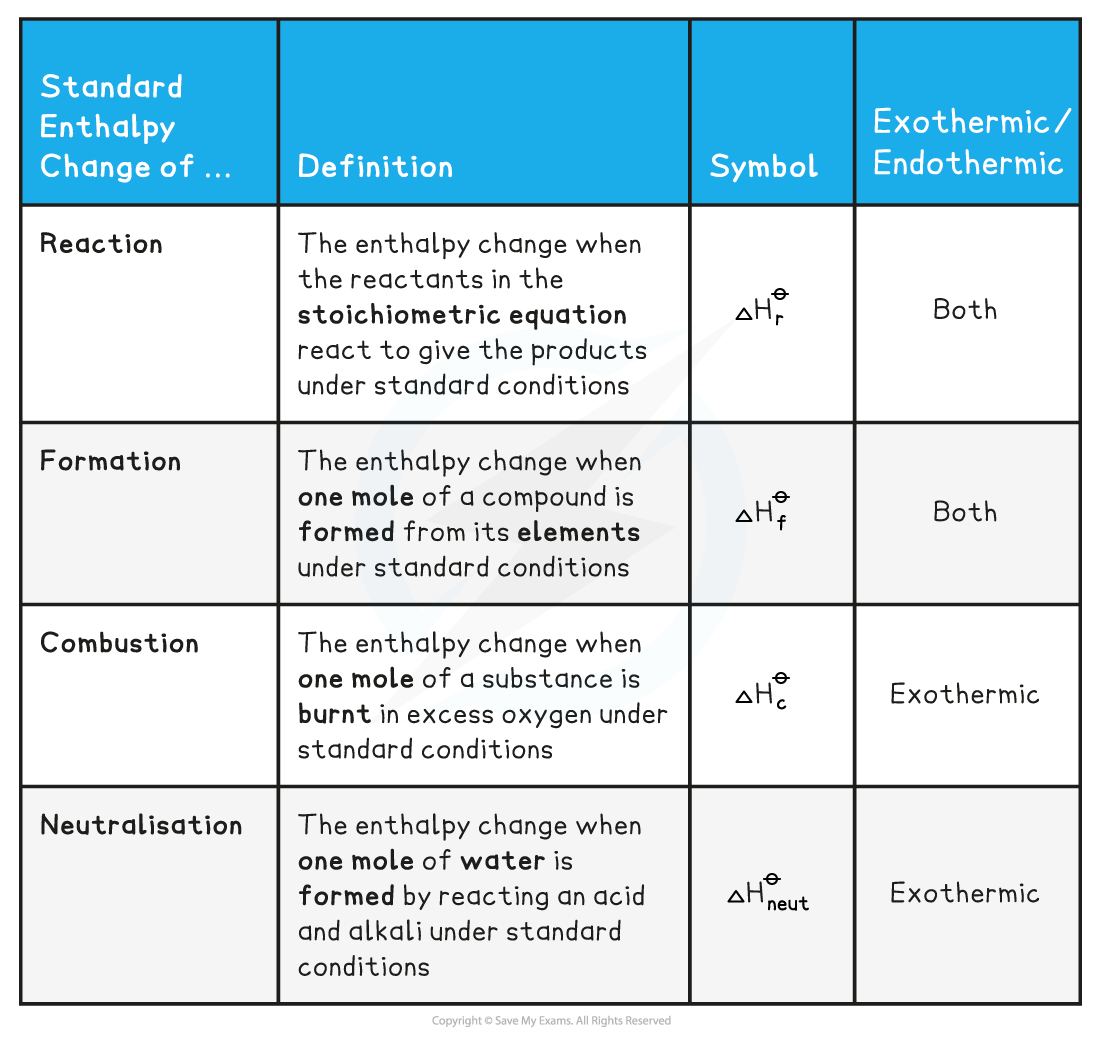
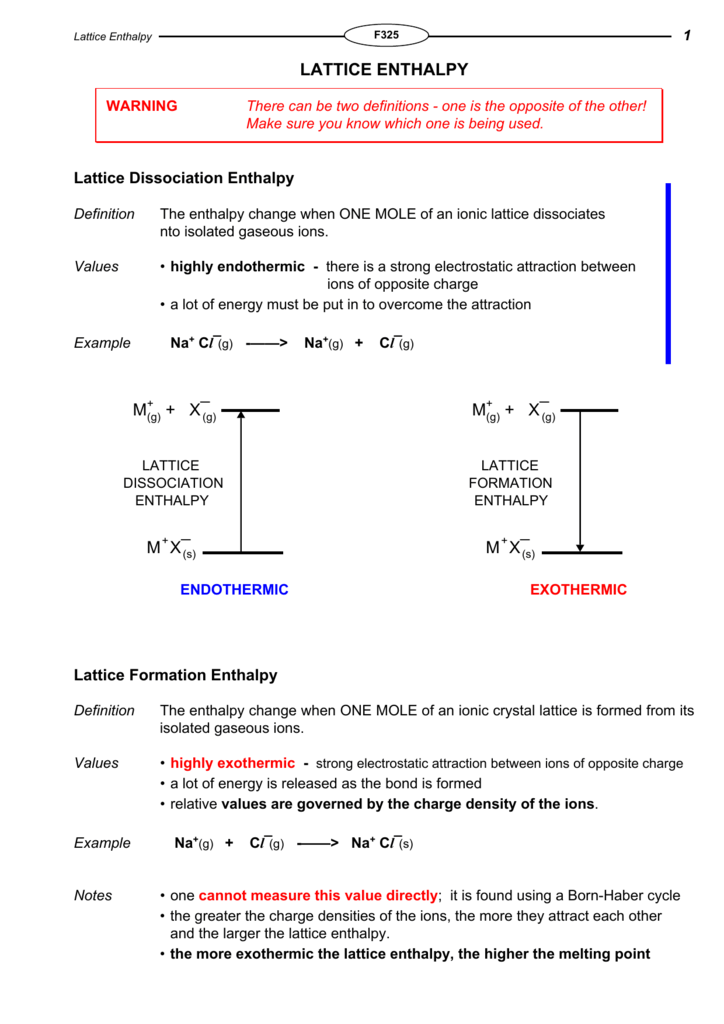
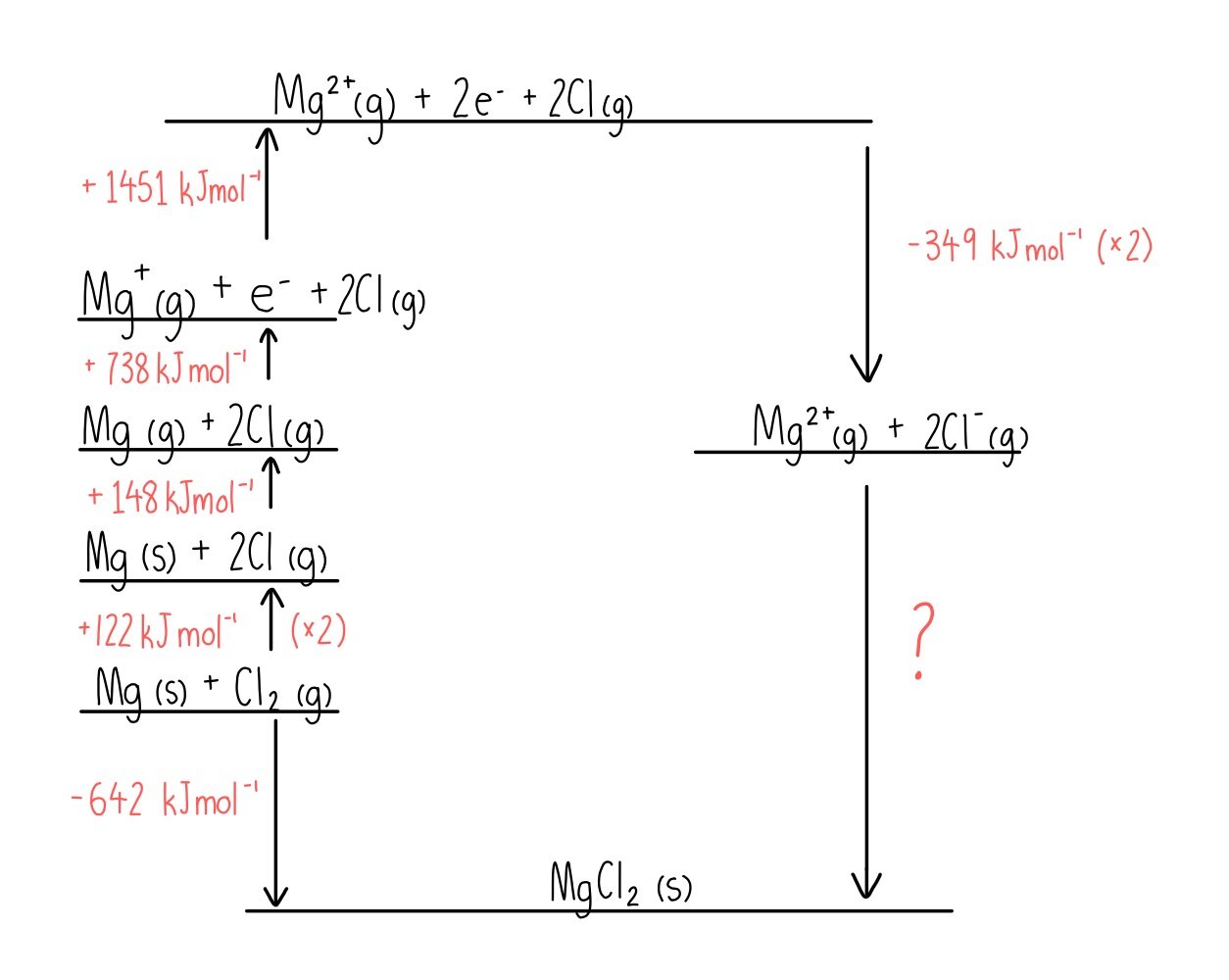
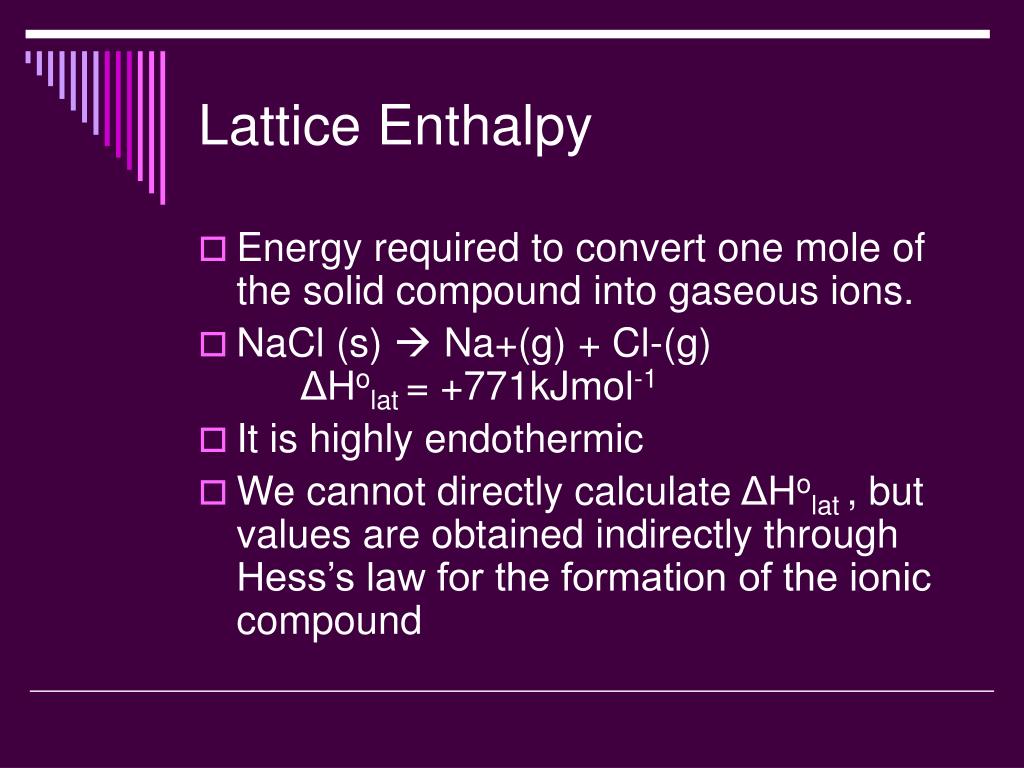
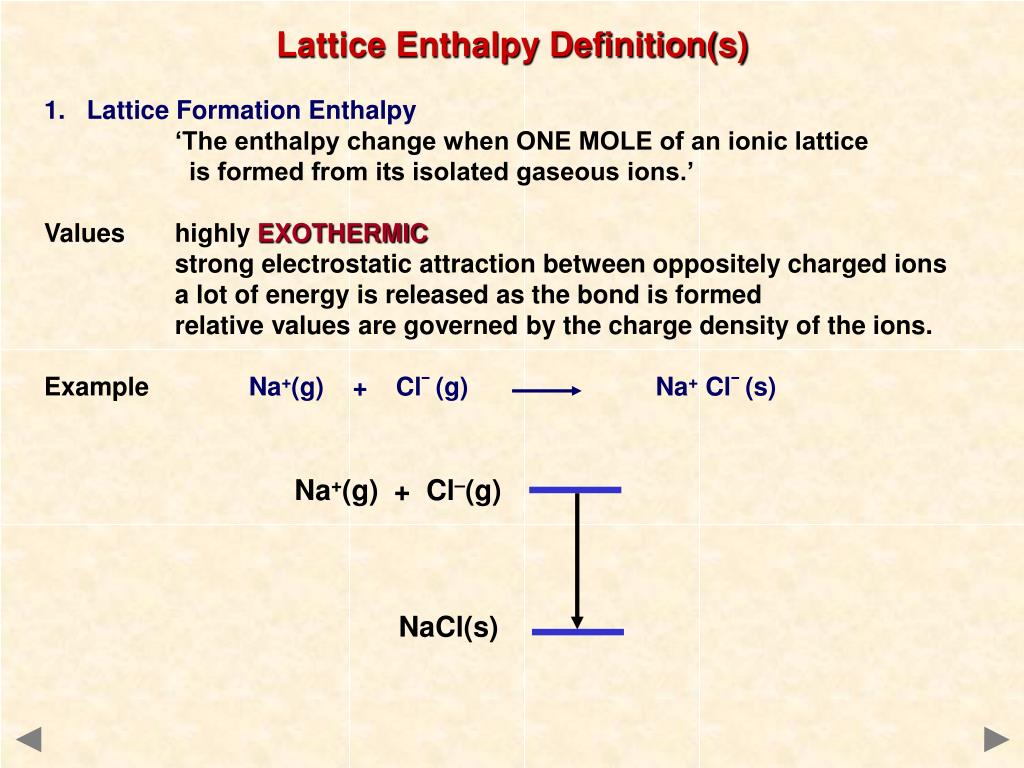
Enthalpy Of Lattice Formation - The greater the lattice enthalpy, the. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions.
Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The greater the lattice enthalpy, the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions.
Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The greater the lattice enthalpy, the. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions.
IB DP Chemistry SL复习笔记5.1.2 Standard Enthalpy Change翰林国际教育
The greater the lattice enthalpy, the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous.
lattice enthalpy
Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The greater the lattice enthalpy, the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous.
Lattice Enthalpy* — the science sauce
The greater the lattice enthalpy, the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic.
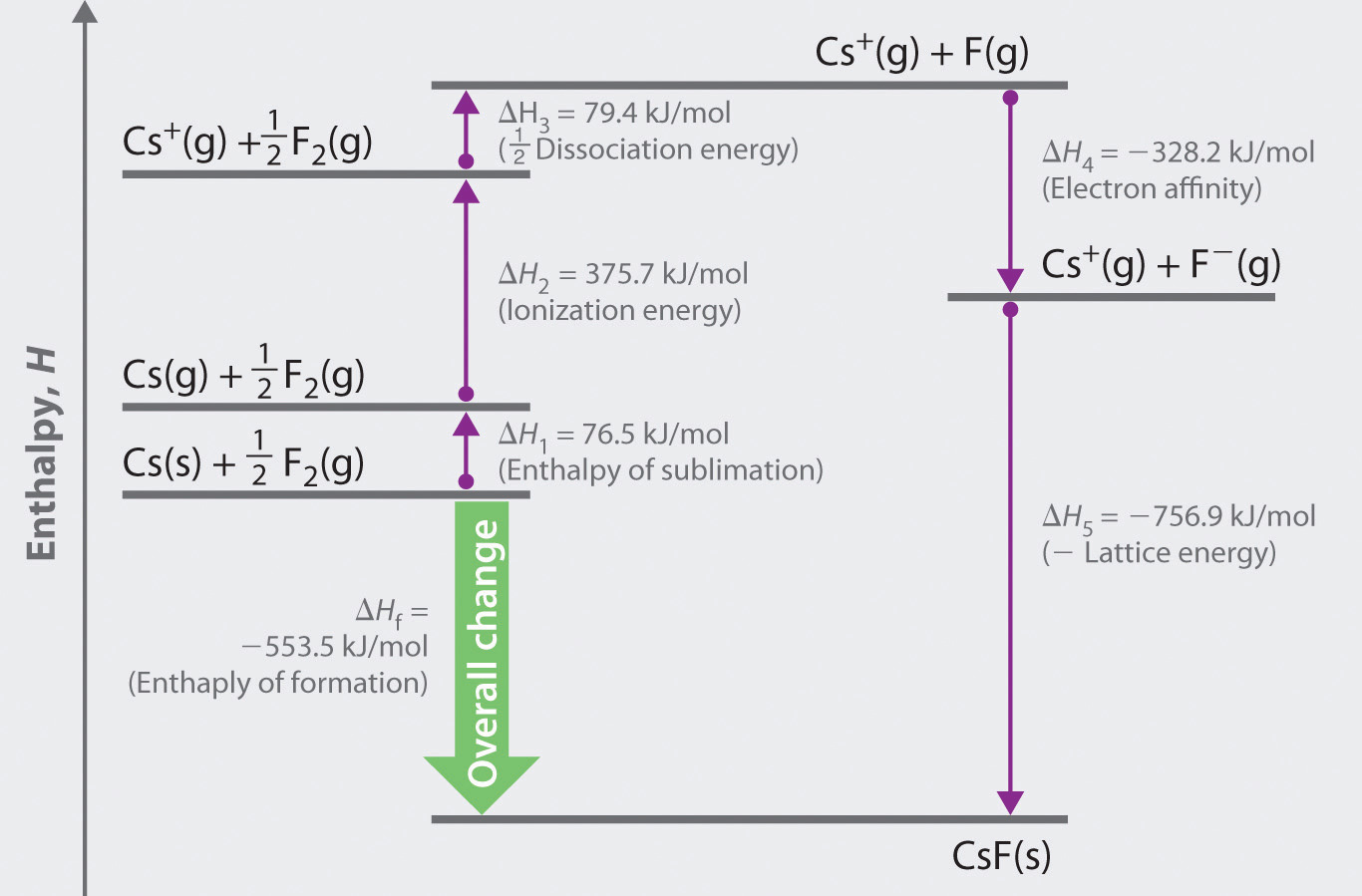
PPT 15.2 BornHaber Cycle PowerPoint Presentation ID154554
The greater the lattice enthalpy, the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and.
PPT Lattice enthalpy PowerPoint Presentation, free download ID6559902
Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The greater the lattice enthalpy,.
PPT Molecular Interactions PowerPoint Presentation, free download
Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. The greater the lattice enthalpy,.
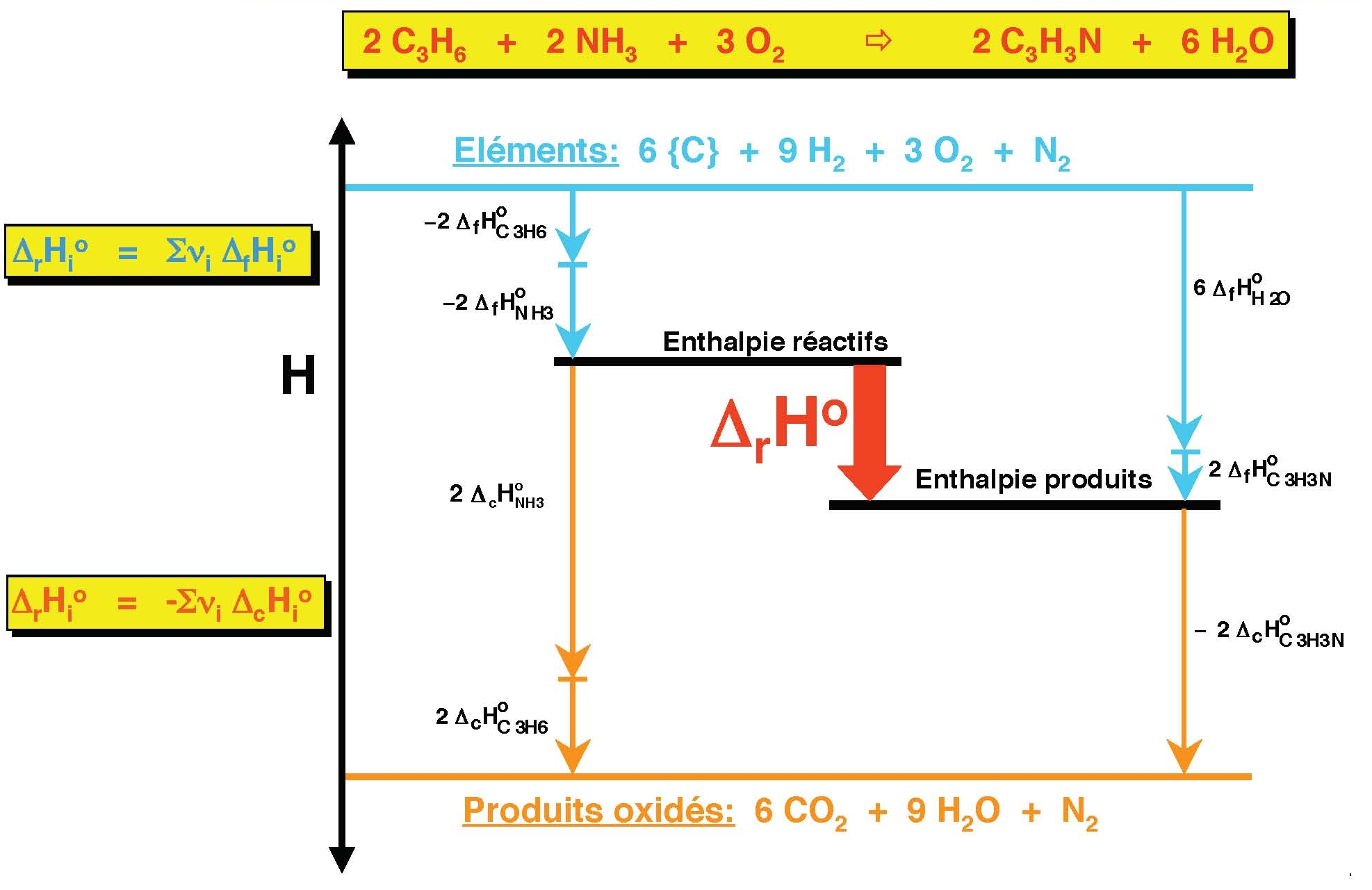
A comparison between the Enthalpy of formation of MgO acquired via a
Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The greater the lattice enthalpy, the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and.
PPT Lattice enthalpy PowerPoint Presentation, free download ID6559902
Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. The greater the lattice enthalpy, the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous.
Lattice Enthalpy* — the science sauce
The greater the lattice enthalpy, the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic.
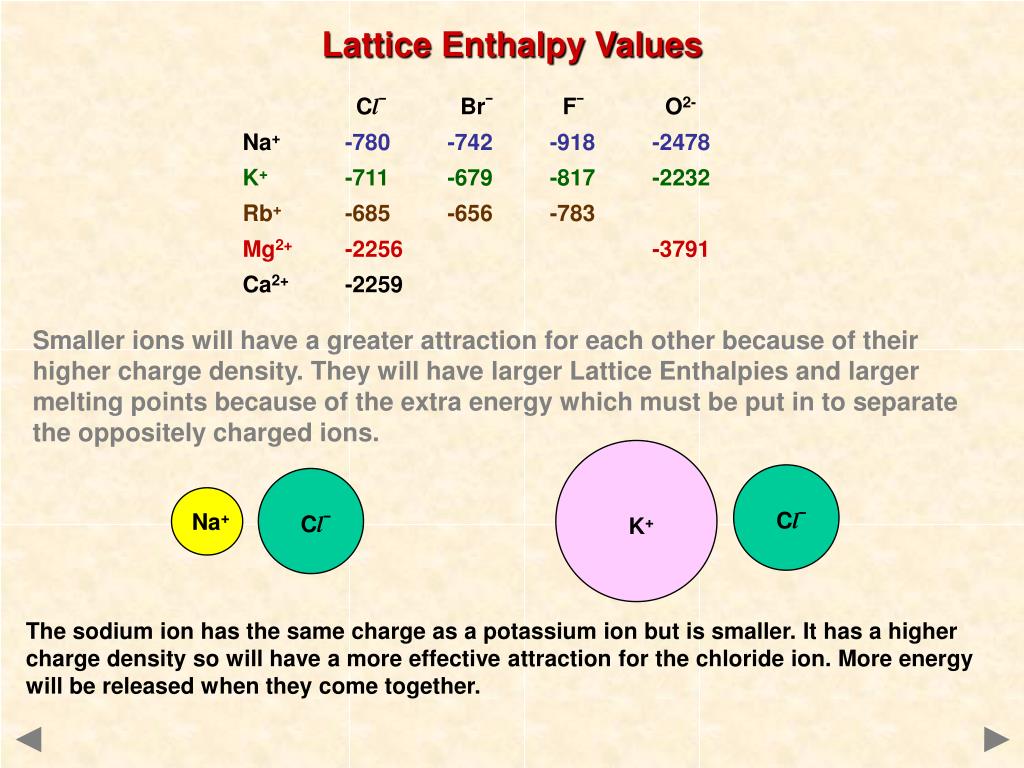
Lattice Energies in Ionic Solids
The greater the lattice enthalpy, the. Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous.
Lattice Enthalpy Is A Measure Of The Strength Of The Forces Between The Ions In An Ionic Solid.
The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its separated gaseous ions. Sometimes questions will give the enthalpy of lattice dissociation which has the opposite sign and the. The greater the lattice enthalpy, the.