Gas Laws Formula Sheet
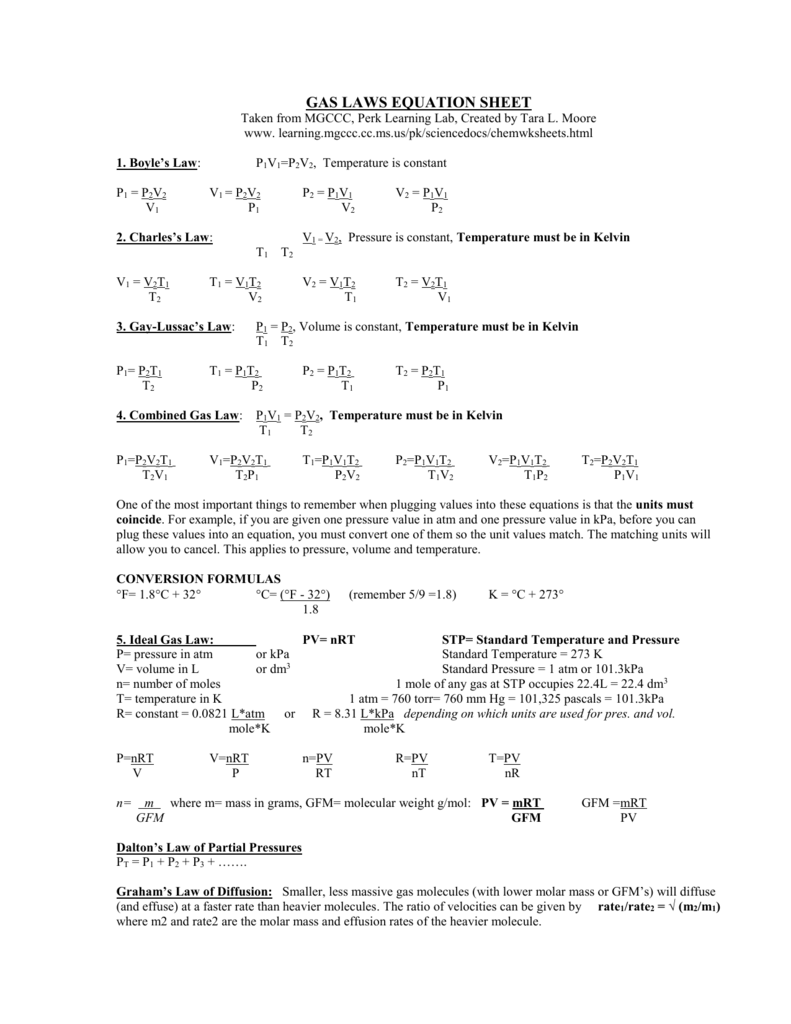
Gas Laws Formula Sheet - The distance between particles is great. Gas laws cheat sheet stp is 1 atm and 0 c k = 273 + c (change all temp to kelvin!!!!) 1 atm = 760 mmhg or 760 torr 1000 ml=1 l. Last updated 23rd november, 2020. Gas particles are neither attracted to nor. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. Gases consist of large numbers of tiny particles, which have mass. The rate of effusion/diffusion of two gases (a.
The distance between particles is great. Gases consist of large numbers of tiny particles, which have mass. Last updated 23rd november, 2020. Gas laws cheat sheet stp is 1 atm and 0 c k = 273 + c (change all temp to kelvin!!!!) 1 atm = 760 mmhg or 760 torr 1000 ml=1 l. Gas particles are neither attracted to nor. The rate of effusion/diffusion of two gases (a. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”.
The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. The rate of effusion/diffusion of two gases (a. Gas particles are neither attracted to nor. Gases consist of large numbers of tiny particles, which have mass. The distance between particles is great. Gas laws cheat sheet stp is 1 atm and 0 c k = 273 + c (change all temp to kelvin!!!!) 1 atm = 760 mmhg or 760 torr 1000 ml=1 l. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. Last updated 23rd november, 2020.
Exercises Sections 10.3, 10.4 The Gas Laws; The IdealGas Equation
Gas particles are neither attracted to nor. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. Gas laws cheat sheet stp is 1 atm and 0 c k = 273 + c (change all temp to kelvin!!!!) 1 atm = 760 mmhg or 760 torr 1000 ml=1 l. The ideal gas law relates the pressure, temperature,.
Gas Laws cheat sheet.docx Google Docs Gay Lussac, Charles Law, Google
The distance between particles is great. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Last updated 23rd november, 2020. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. Gas particles are neither attracted to nor.
Chemistry lessons, Science notes, High school science
Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. The distance between particles is great. Last updated 23rd november, 2020. The rate of effusion/diffusion of two gases (a. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”.
GAS LAWS Equation Sheet
The distance between particles is great. Gas particles are neither attracted to nor. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. The rate of effusion/diffusion of two gases (a. Gases consist of large numbers of tiny particles, which have mass.
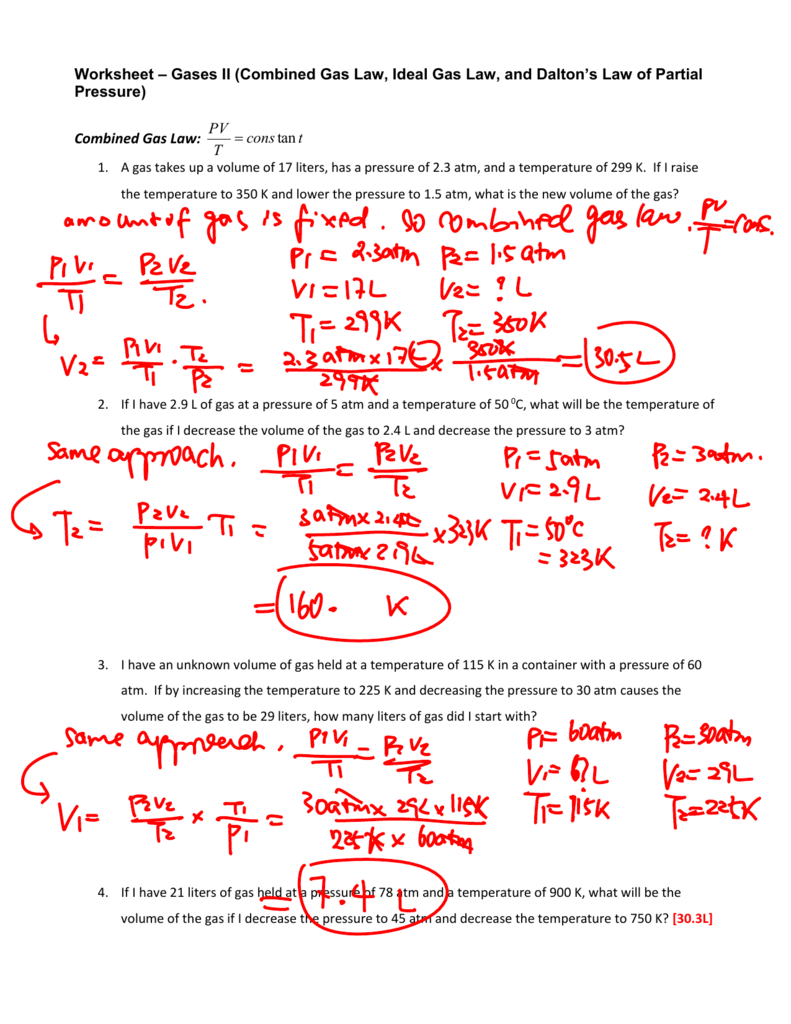
Worksheet Gas Laws II Answers
Gas particles are neither attracted to nor. Gases consist of large numbers of tiny particles, which have mass. The rate of effusion/diffusion of two gases (a. Last updated 23rd november, 2020. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3.
Combined Gas Law Definition, Formula, Examples
Gas laws cheat sheet stp is 1 atm and 0 c k = 273 + c (change all temp to kelvin!!!!) 1 atm = 760 mmhg or 760 torr 1000 ml=1 l. Gases consist of large numbers of tiny particles, which have mass. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. The ideal gas law.
Combined Gas Law — Overview & Calculations Expii
The distance between particles is great. Gases consist of large numbers of tiny particles, which have mass. The rate of effusion/diffusion of two gases (a. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”.
Gas Laws Practice Test
The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. The rate of effusion/diffusion of two gases (a. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. Gas particles are neither attracted to nor. Gases consist of large numbers of tiny particles, which have mass.
Gas Law Formulas and Equations College Chemistry Study Guide YouTube
Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3. The distance between particles is great. The rate of effusion/diffusion of two gases (a. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Last updated 23rd november, 2020.
Gas Particles Are Neither Attracted To Nor.
Gases consist of large numbers of tiny particles, which have mass. The distance between particles is great. Last updated 23rd november, 2020. The ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”.
The Rate Of Effusion/Diffusion Of Two Gases (A.
Gas laws cheat sheet stp is 1 atm and 0 c k = 273 + c (change all temp to kelvin!!!!) 1 atm = 760 mmhg or 760 torr 1000 ml=1 l. Dalton's law of partial p total =p 1 +p 2 ‐pressure +p 3.