Gmp Date Format
Gmp Date Format - All instruction documents should have the effective date printed or stamped on them. When a document has been revised, systems must be operated to prevent. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. All dates should be expressed in a format. 1.4 the preferred format for the handwritten date on documents is the format: 2 digit day, three character month, 4 digit year.
All dates should be expressed in a format. When a document has been revised, systems must be operated to prevent. 1.4 the preferred format for the handwritten date on documents is the format: All instruction documents should have the effective date printed or stamped on them. 2 digit day, three character month, 4 digit year. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be.
All dates should be expressed in a format. 1.4 the preferred format for the handwritten date on documents is the format: 2 digit day, three character month, 4 digit year. All instruction documents should have the effective date printed or stamped on them. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. When a document has been revised, systems must be operated to prevent.
GMP
All instruction documents should have the effective date printed or stamped on them. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. All dates should be expressed in a format. When a document has been revised, systems must be operated to prevent. 1.4 the preferred format for.
GMP Documents for Pharmaceutical Company
All dates should be expressed in a format. 2 digit day, three character month, 4 digit year. All instruction documents should have the effective date printed or stamped on them. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. When a document has been revised, systems must.
What is GMP standard in pharmaceutical manufacturing
Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. 2 digit day, three character month, 4 digit year. All instruction documents should have the effective date printed or stamped on them. 1.4 the preferred format for the handwritten date on documents is the format: When a document.
GMP Annex 1 2022 Update Breakdown Part 1
Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. All dates should be expressed in a format. All instruction documents should have the effective date printed or stamped on them. 1.4 the preferred format for the handwritten date on documents is the format: When a document has.
GMP vs GLP Understand the Key Differences SOS Inventory
When a document has been revised, systems must be operated to prevent. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. 2 digit day, three character month, 4 digit year. All dates should be expressed in a format. 1.4 the preferred format for the handwritten date on.
HMA Agro IPO Details GMP, Date, Price, Review, Allotment
1.4 the preferred format for the handwritten date on documents is the format: All dates should be expressed in a format. All instruction documents should have the effective date printed or stamped on them. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. 2 digit day, three.
A Review on Good Manufacturing Practice (GMP) for Medicinal Products
2 digit day, three character month, 4 digit year. 1.4 the preferred format for the handwritten date on documents is the format: Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. When a document has been revised, systems must be operated to prevent. All dates should be.
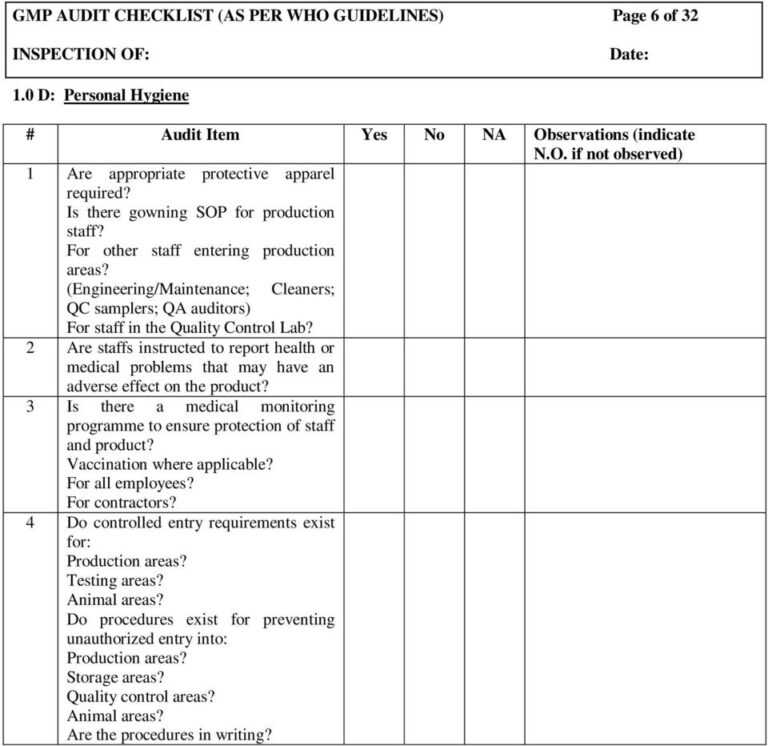
Gmp Audit Checklist (As Per Who Guidelines) Page 1 Of 32 pertaining to
1.4 the preferred format for the handwritten date on documents is the format: 2 digit day, three character month, 4 digit year. When a document has been revised, systems must be operated to prevent. All dates should be expressed in a format. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other.
GMP Template Doc Template pdfFiller
When a document has been revised, systems must be operated to prevent. 1.4 the preferred format for the handwritten date on documents is the format: Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. 2 digit day, three character month, 4 digit year. All instruction documents should.
(PDF) Gmp Requirements DOKUMEN.TIPS
2 digit day, three character month, 4 digit year. All dates should be expressed in a format. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. 1.4 the preferred format for the handwritten date on documents is the format: All instruction documents should have the effective date.
1.4 The Preferred Format For The Handwritten Date On Documents Is The Format:
All dates should be expressed in a format. 2 digit day, three character month, 4 digit year. When a document has been revised, systems must be operated to prevent. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be.