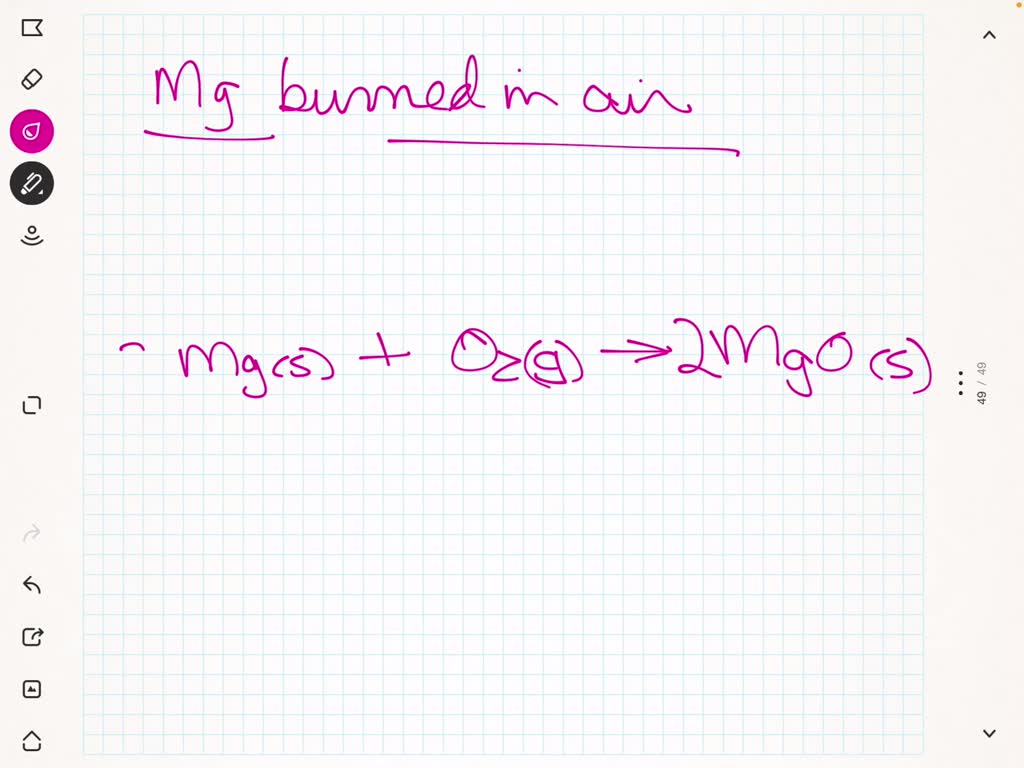Magnesium Oxygen Word Equation
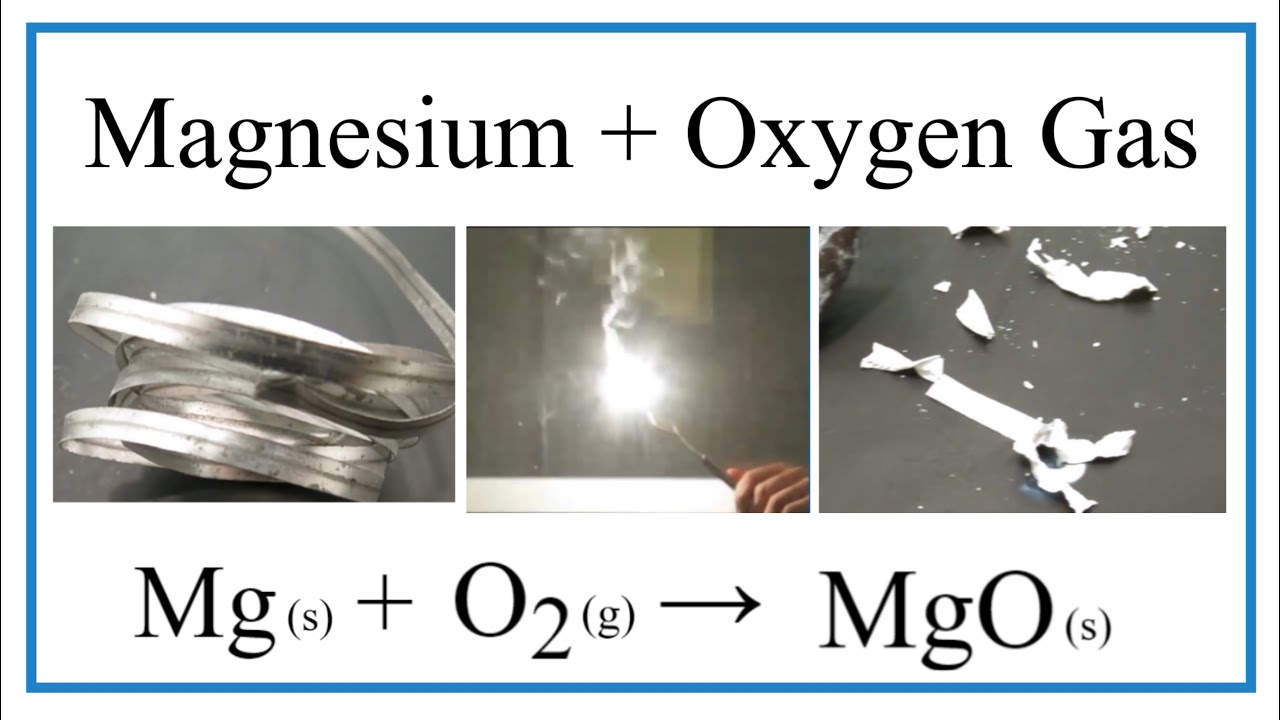
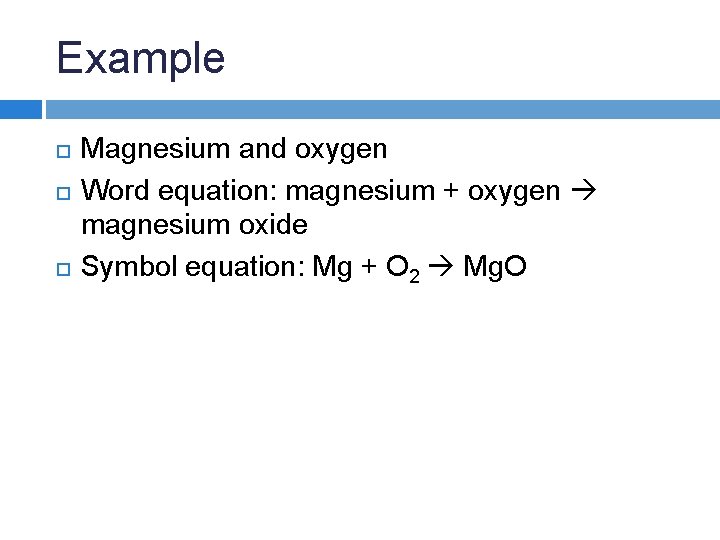
Magnesium Oxygen Word Equation - For example, magnesium and oxygen in the air react to produce magnesium oxide. Magnesium + dioxygen = magnesium oxide. Magnesium + oxygen = magnesium + dioxygen. This is because in a chemical reaction, the reactants (in this case, magnesium. The word and symbol equations are: Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. Magnesium + oxygen = magnesium oxide. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen.
Magnesium + dioxygen = magnesium oxide. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. This is because in a chemical reaction, the reactants (in this case, magnesium. The word and symbol equations are: For example, magnesium and oxygen in the air react to produce magnesium oxide. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Magnesium + oxygen = magnesium oxide. Magnesium + oxygen = magnesium + dioxygen. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and.
Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. For example, magnesium and oxygen in the air react to produce magnesium oxide. Magnesium + dioxygen = magnesium oxide. Magnesium + oxygen = magnesium oxide. Magnesium + oxygen = magnesium + dioxygen. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. This is because in a chemical reaction, the reactants (in this case, magnesium. The word and symbol equations are: Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and.
Simple Composition Reaction of magnesium and oxygen YouTube
Magnesium + oxygen = magnesium oxide. For example, magnesium and oxygen in the air react to produce magnesium oxide. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. The word and symbol equations are: Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and.
SOLVED A strip of Magnesium ribbon should be held in the flame of a
One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Magnesium + oxygen = magnesium oxide. The word.
Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen
For example, magnesium and oxygen in the air react to produce magnesium oxide. Magnesium + oxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Since one molecule of o₂ contains two oxygen atoms, we need.
Fun Word Equation For Magnesium And Oxygen Mlt Dimensions Table
The word and symbol equations are: Magnesium + dioxygen = magnesium oxide. Magnesium + oxygen = magnesium oxide. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. For example, magnesium and oxygen in the air react to produce magnesium oxide.
What happens when Magnesium oxide is dissolved in water? Write a word
The word and symbol equations are: One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Magnesium + dioxygen = magnesium oxide. Magnesium + oxygen = magnesium + dioxygen. Magnesium + oxygen = magnesium oxide.
CHEMICAL EQUATIONS Chemical equations You are expected to
One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium oxide. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and.
Solved Balanced equation for magnesium burning in oxygen
Magnesium + oxygen = magnesium oxide. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Magnesium + dioxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. The word and symbol equations are:
Write a word equation to show what happens when magnesium burns in
Magnesium + oxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Magnesium + oxygen = magnesium.
Reactions & Formulas
For example, magnesium and oxygen in the air react to produce magnesium oxide. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. This is because in a chemical reaction, the reactants (in this case, magnesium. One mole of magnesium [mg] and two moles of oxygen [o] react to form one.
magnesium oxygen reaction
For example, magnesium and oxygen in the air react to produce magnesium oxide. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium + dioxygen. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of..
Mg + O2 = Mgo Is A Synthesis Reaction Where Two Moles Of Magnesium [Mg] And.
Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. The word and symbol equations are: Magnesium + oxygen = magnesium + dioxygen. This is because in a chemical reaction, the reactants (in this case, magnesium.
Magnesium + Oxygen = Magnesium Oxide.
Magnesium + dioxygen = magnesium oxide. For example, magnesium and oxygen in the air react to produce magnesium oxide. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen.