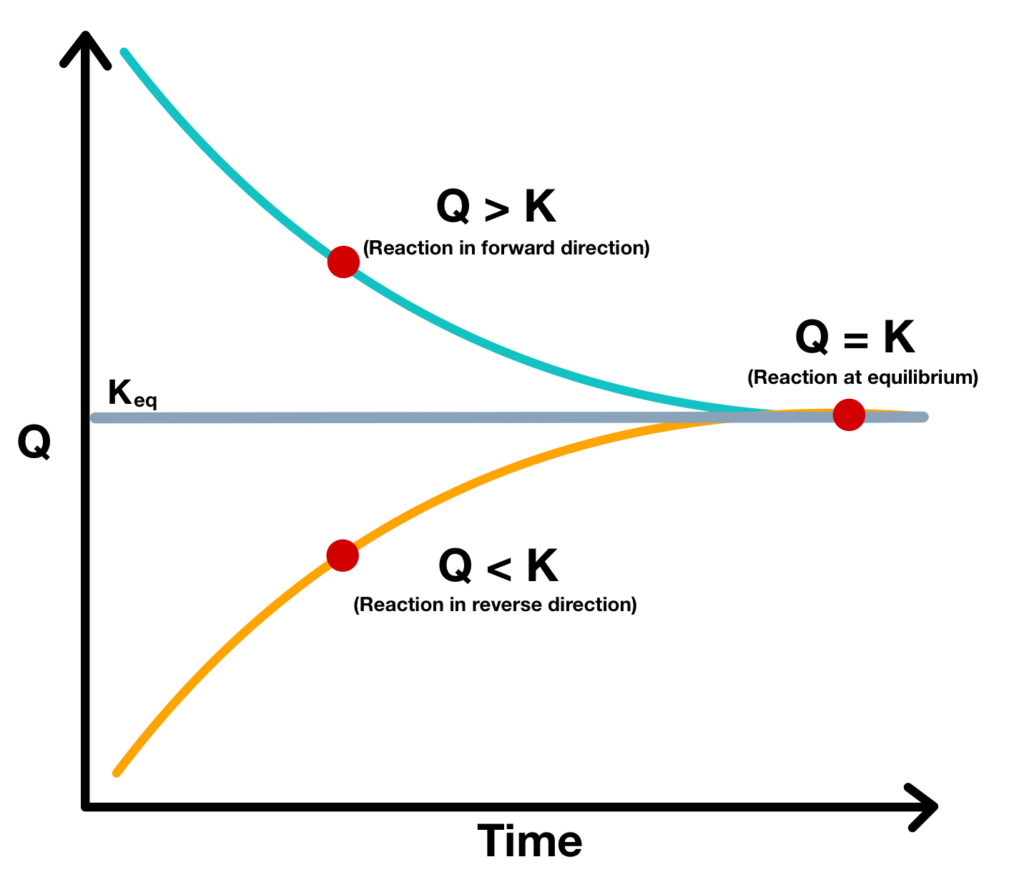
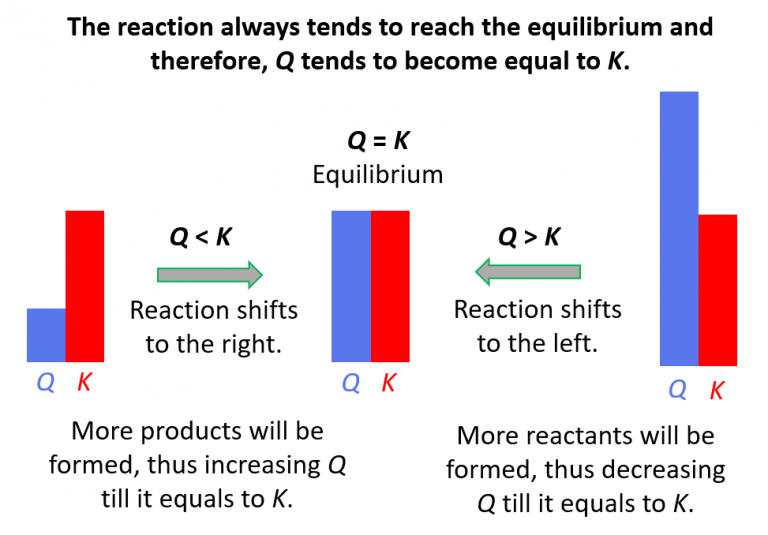
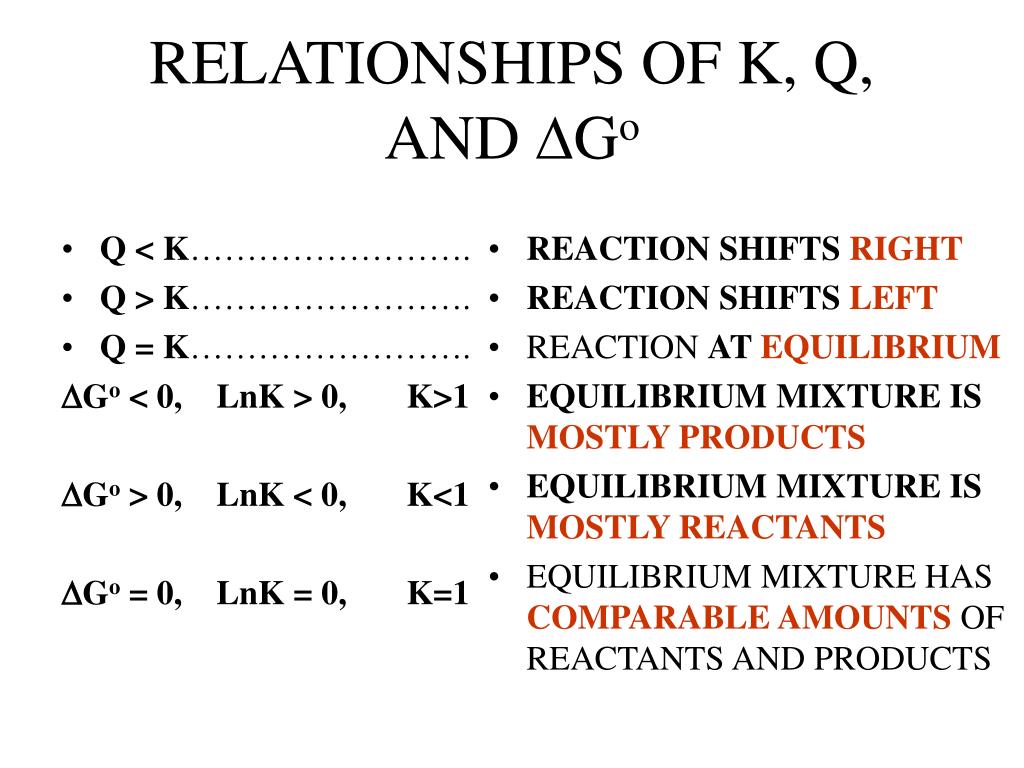
Relationship Between Q And K
Relationship Between Q And K - Q can be used to determine which direction a reaction will shift to reach equilibrium. Q and k are used to describe the state of equilibrium in a chemical reaction. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. If k > q, a reaction will proceed forward, converting. The equilibrium constant is denoted by the letter “k” in. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. K represents the equilibrium constant, which is.
K represents the equilibrium constant, which is. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. The equilibrium constant is denoted by the letter “k” in. Q can be used to determine which direction a reaction will shift to reach equilibrium. Q and k are used to describe the state of equilibrium in a chemical reaction. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. If k > q, a reaction will proceed forward, converting.
K represents the equilibrium constant, which is. If k > q, a reaction will proceed forward, converting. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q can be used to determine which direction a reaction will shift to reach equilibrium. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. The equilibrium constant is denoted by the letter “k” in. Q and k are used to describe the state of equilibrium in a chemical reaction.
Reaction Quotient (K) and Equilibrium Constant (K) Problems & Examples
If k > q, a reaction will proceed forward, converting. The equilibrium constant is denoted by the letter “k” in. Q and k are used to describe the state of equilibrium in a chemical reaction. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q can be used to determine which direction a.
Reaction Quotient (Q) Equation, Calculation, Types, Units
The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q and k are used to describe the state of equilibrium in a chemical reaction. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. Q can be used to determine which.
What Is Kc In Chemistry slideshare
K represents the equilibrium constant, which is. If k > q, a reaction will proceed forward, converting. Q and k are used to describe the state of equilibrium in a chemical reaction. The equilibrium constant is denoted by the letter “k” in. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas.
The Difference between Q and Keq (Equilibrium) YouTube
The equilibrium constant is denoted by the letter “k” in. Q can be used to determine which direction a reaction will shift to reach equilibrium. K represents the equilibrium constant, which is. If k > q, a reaction will proceed forward, converting. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction.
Relationship between q k and q for different values of Δ (m = 4
Q can be used to determine which direction a reaction will shift to reach equilibrium. If k > q, a reaction will proceed forward, converting. Q and k are used to describe the state of equilibrium in a chemical reaction. The equilibrium constant is denoted by the letter “k” in. K represents the equilibrium constant, which is.
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
K represents the equilibrium constant, which is. Q and k are used to describe the state of equilibrium in a chemical reaction. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q can be used to determine which direction a reaction will shift to reach equilibrium. The equilibrium constant is denoted by the.
Explain the Difference Between K Kp and Q LarryhasPark
Q can be used to determine which direction a reaction will shift to reach equilibrium. If k > q, a reaction will proceed forward, converting. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. K represents the equilibrium constant, which is. Q and k are used to describe the state of equilibrium in.
Establish relationship between Kp Kc?
The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. If k > q, a reaction will proceed forward, converting. K represents the equilibrium constant, which is. The equilibrium constant is denoted by the letter “k” in. Q and k are used to describe the state of equilibrium in a chemical reaction.
Reaction Quotient Q Chemistry Steps
The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. The equilibrium constant is denoted by the letter “k” in. If k > q, a reaction will proceed forward, converting. K represents the equilibrium constant, which is. Q and k are used to describe the state of equilibrium in a chemical reaction.
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID
Q and k are used to describe the state of equilibrium in a chemical reaction. Q can be used to determine which direction a reaction will shift to reach equilibrium. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction. The equilibrium constant is denoted by the letter.
If K > Q, A Reaction Will Proceed Forward, Converting.
Q can be used to determine which direction a reaction will shift to reach equilibrium. The reaction constant or reaction quotient is denoted by the letter “q” in any equilibrium reaction. Q and k are used to describe the state of equilibrium in a chemical reaction. The main difference between \(k\) and \(q\) is that \(k\) describes a reaction that is at equilibrium, whereas \(q\) describes a reaction.
K Represents The Equilibrium Constant, Which Is.
The equilibrium constant is denoted by the letter “k” in.