Relyvrio Start Form
Relyvrio Start Form - Start saving time today by filling out this form electronically. For formulary information please visit www.myprime.com. To prescribe relyvrio and enroll your patient in the act support program, follow these 4 steps: Attached is a prior authorization request form. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Be sure to note optum frontier therapies as your preferred pharmacy. Now you can get responses to drug prior authorization. Download, complete and sign the necessary therapy enrollment form. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Have your patient read the patient authorization and.
For formulary information please visit www.myprime.com. Be sure to note optum frontier therapies as your preferred pharmacy. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. To prescribe relyvrio and enroll your patient in the act support program, follow these 4 steps: Attached is a prior authorization request form. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Download, complete and sign the necessary therapy enrollment form. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Have your patient read the patient authorization and. Now you can get responses to drug prior authorization.
To prescribe relyvrio and enroll your patient in the act support program, follow these 4 steps: Now you can get responses to drug prior authorization. Have your patient read the patient authorization and. Attached is a prior authorization request form. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Be sure to note optum frontier therapies as your preferred pharmacy. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Download, complete and sign the necessary therapy enrollment form. For formulary information please visit www.myprime.com. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024.
October 2022 Foundation eNews Les Turner ALS Foundation
Download, complete and sign the necessary therapy enrollment form. For formulary information please visit www.myprime.com. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Attached is a prior authorization request form. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in.
Relyvrio for ALS ALS News Today
For formulary information please visit www.myprime.com. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Now you can get responses to drug prior authorization. Start saving time today by filling out this form electronically.
Relyvrio服药指南_医伴旅
Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Attached is a prior authorization request form. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Be sure to note optum frontier therapies as your preferred pharmacy. To prescribe relyvrio and enroll your.
Newly Approved ALS Drug Relyvrio To Face Its Next Challenge Pricing
Attached is a prior authorization request form. Be sure to note optum frontier therapies as your preferred pharmacy. Now you can get responses to drug prior authorization. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. For formulary information please visit www.myprime.com.
FDA Approves AMX0035 (Relyvrio) for ALS
Attached is a prior authorization request form. Be sure to note optum frontier therapies as your preferred pharmacy. Now you can get responses to drug prior authorization. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Download, complete and sign the necessary therapy enrollment form.
DailyMed RELYVRIO sodium phenylbutyrate/taurursodiol powder, for
In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Download, complete and sign the necessary therapy enrollment form. For formulary information please visit www.myprime.com. Attached is a prior authorization request form. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated.
New drug to treat ALS passes through FDA despite concerns of efficacy
Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. For formulary information please visit www.myprime.com. To prescribe relyvrio and enroll your patient in the act support program, follow these 4 steps: Now you can get responses.
RELYVRIO sodium phenylbutyrate/taurursodiol powder, for suspension
Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. For formulary information please visit www.myprime.com. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Start saving time today by filling out this form electronically. Be sure to note optum frontier therapies as your preferred pharmacy.
FDA Approves New ALS Drug Relyvrio That Aims to Slow Disease Progression
Start saving time today by filling out this form electronically. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Download, complete and sign the necessary therapy enrollment form. Now you can get responses to drug prior authorization. To prescribe relyvrio and enroll your patient in the act support.
RelyvrioRelyvrio中文说明书,价格,哪里有卖香港济民药业
Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Attached is a prior authorization request form. Now you can get responses to drug prior authorization. Be sure to note optum frontier therapies as your preferred pharmacy. Download, complete and sign the necessary therapy enrollment form.
Attached Is A Prior Authorization Request Form.
For formulary information please visit www.myprime.com. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Start saving time today by filling out this form electronically. Now you can get responses to drug prior authorization.
Be Sure To Note Optum Frontier Therapies As Your Preferred Pharmacy.
In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Download, complete and sign the necessary therapy enrollment form. To prescribe relyvrio and enroll your patient in the act support program, follow these 4 steps:





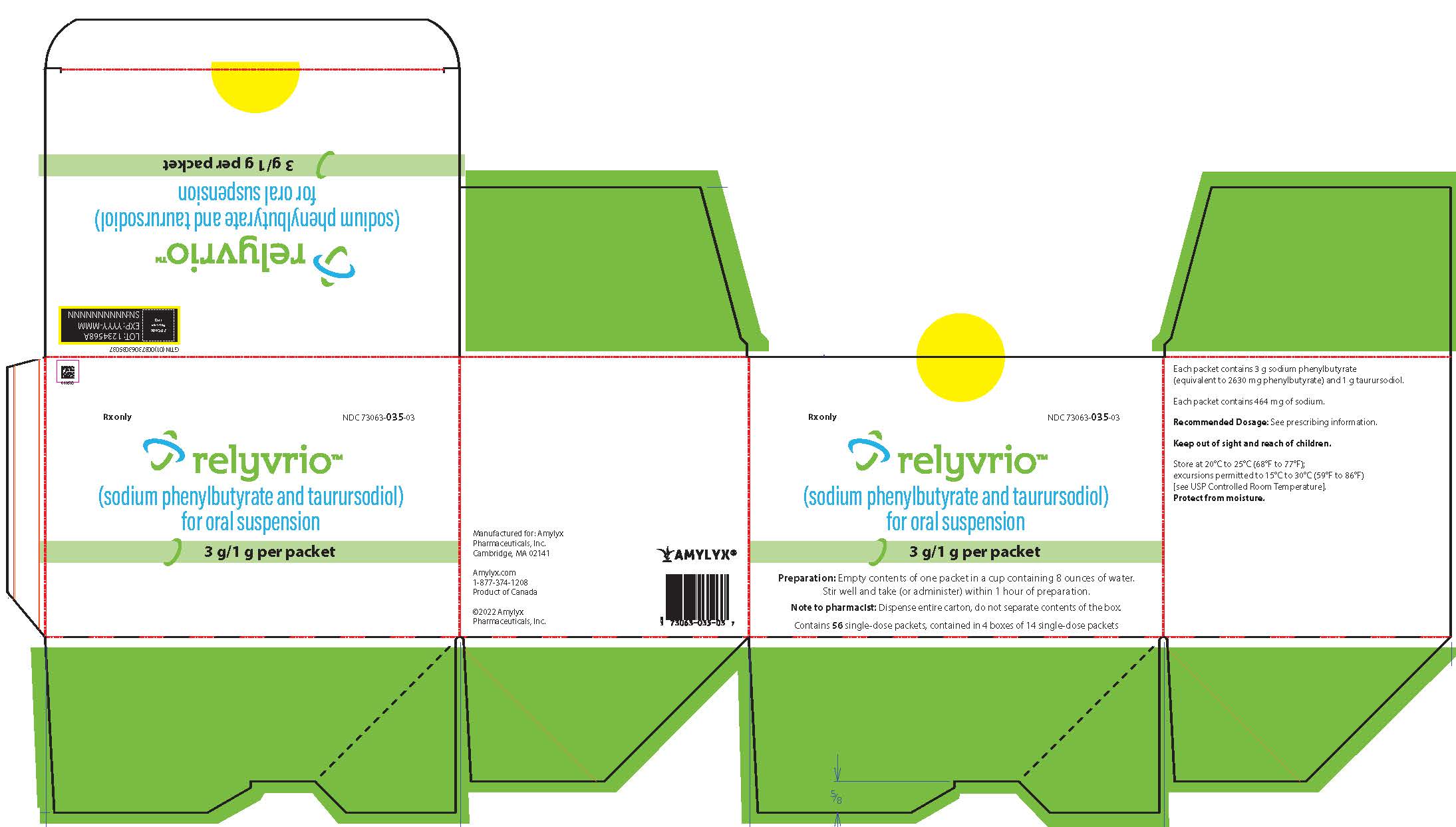

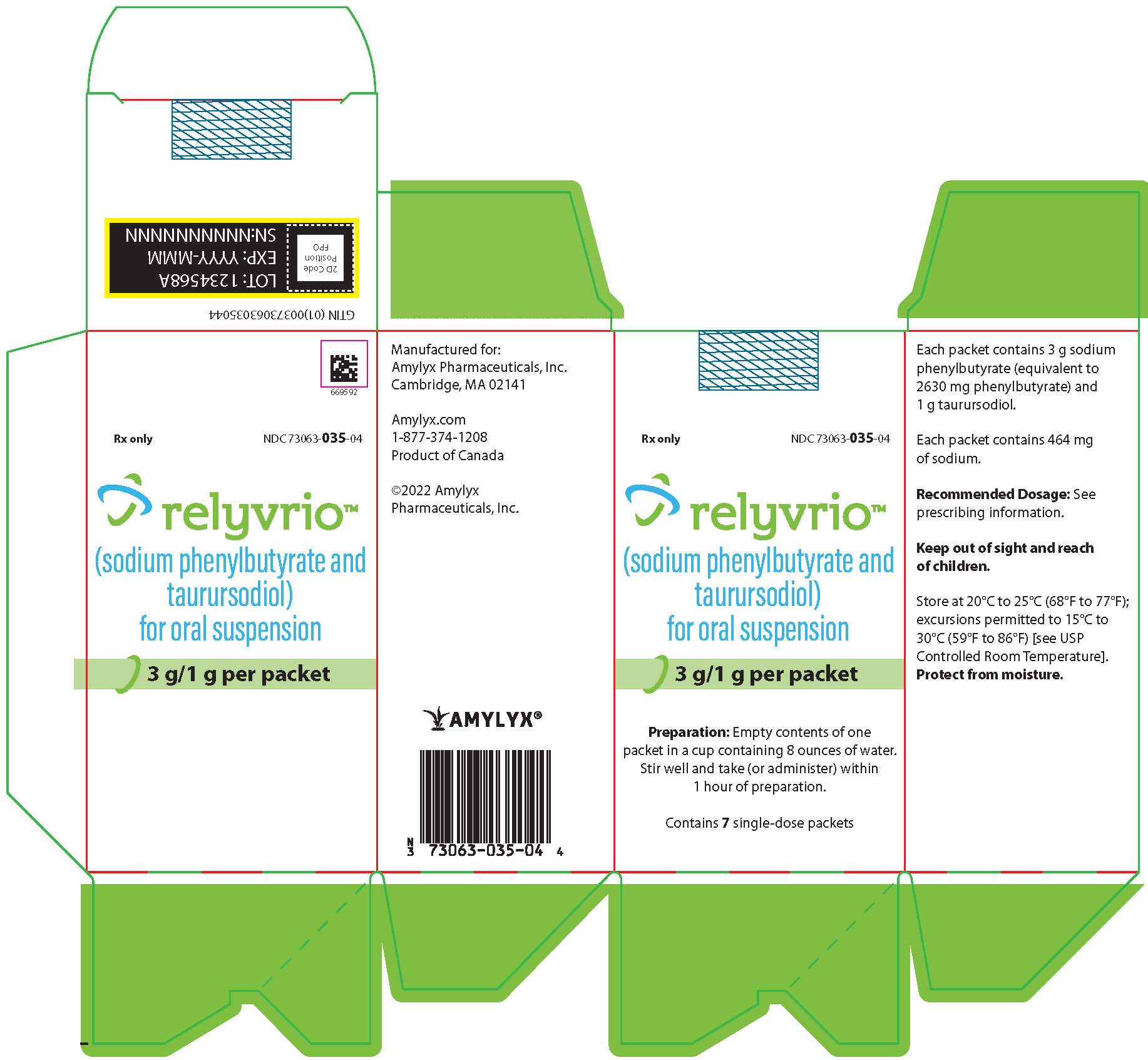
:max_bytes(150000):strip_icc()/relyvrio-credit-Amylyx-Pharmaceuticals-d5ab86a7fdf84d65b8f54eed10a94ee8.jpg)
