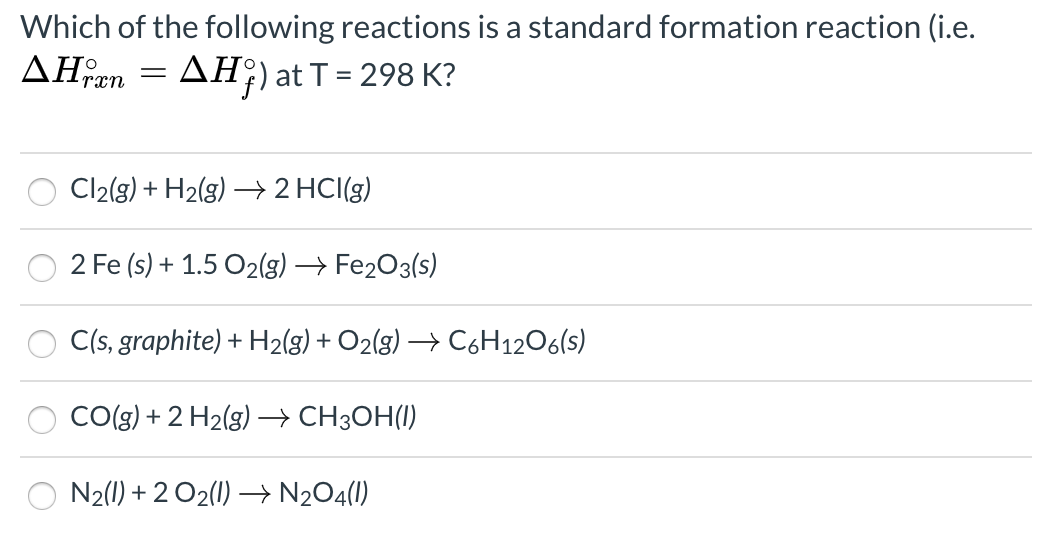Standard Formation Reaction Of Liquid Ethanol
Standard Formation Reaction Of Liquid Ethanol - 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Determine the standard enthalpy of formation for ethylene glycol. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. What is the standard enthalpy of formation for ethanol c 2h 5oh? 1) the first thing to do is look up standard enthalpies of formation for the.
1) the first thing to do is look up standard enthalpies of formation for the. Determine the standard enthalpy of formation for ethylene glycol. What is the standard enthalpy of formation for ethanol c 2h 5oh? 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 1) the first thing to do is look up standard enthalpies of formation for the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. What is the standard enthalpy of formation for ethanol c 2h 5oh? The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Determine the standard enthalpy of formation for ethylene glycol. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of.
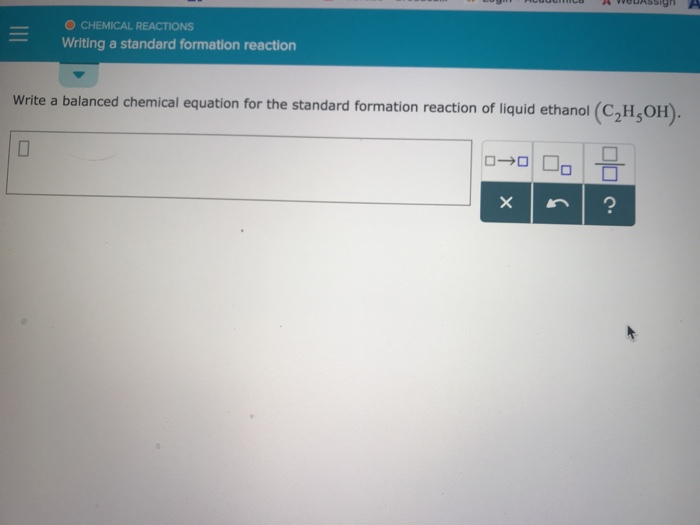
Solved O CHEMICAL REACTIONS Writing a standard formation
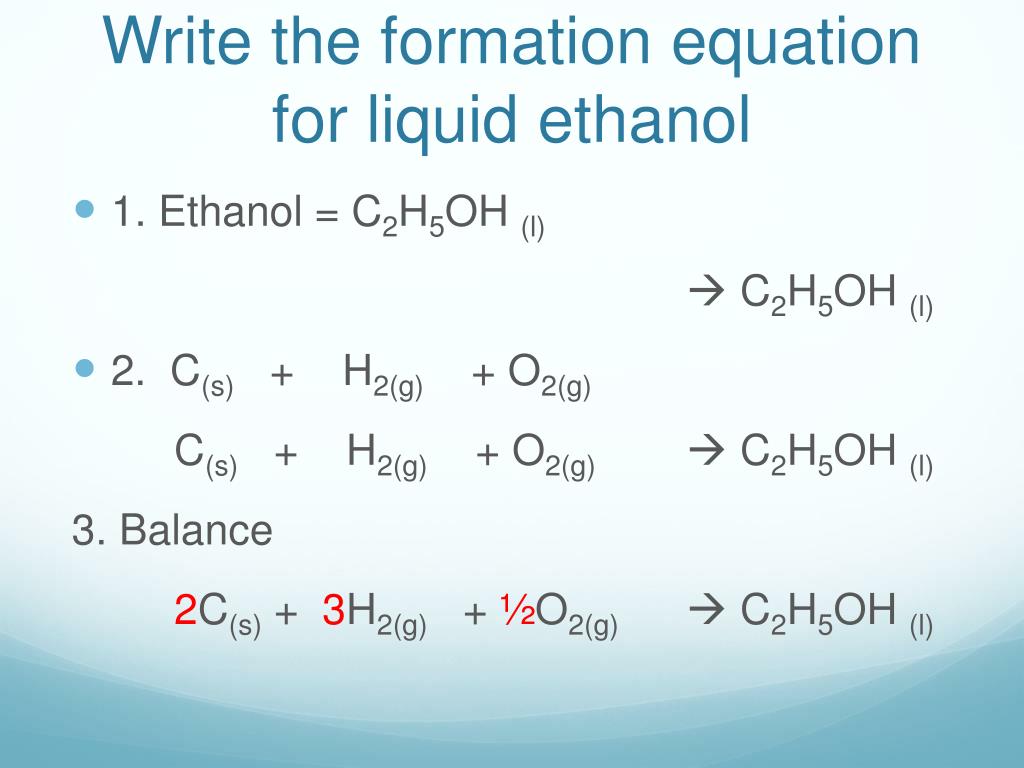
Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all.
Standard Enthalpy of Formation and Formation Reactions OpenStax
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. What is the standard enthalpy of formation for ethanol c 2h 5oh? The standard enthalpy.
SOLVED a) Write a balanced chemical equation for the standard enthalpy
Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. The standard.
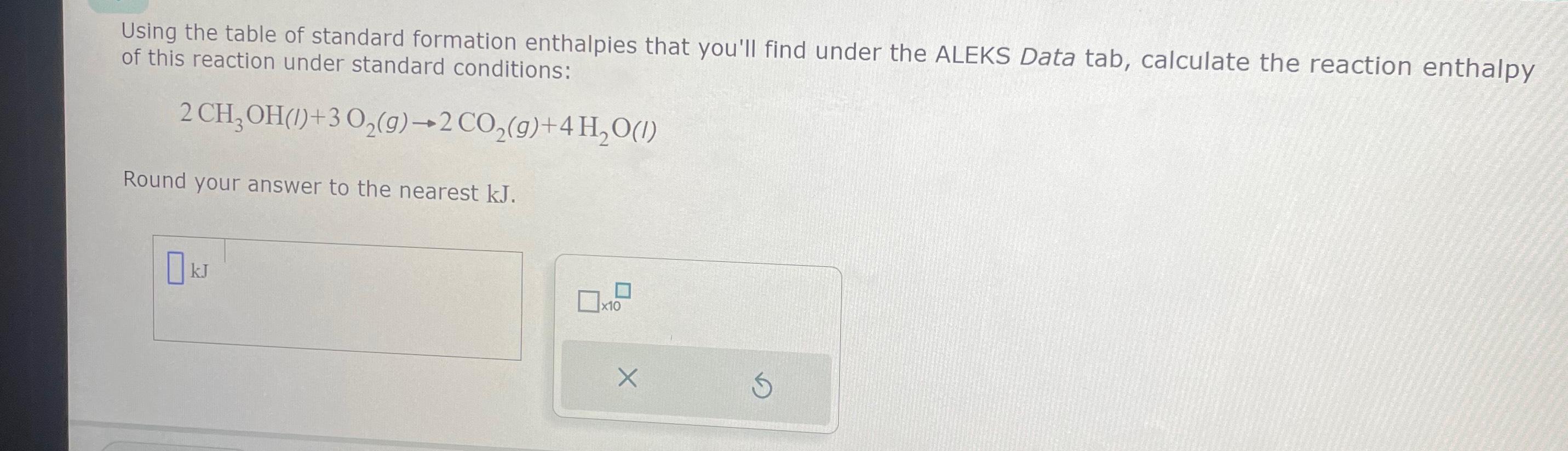
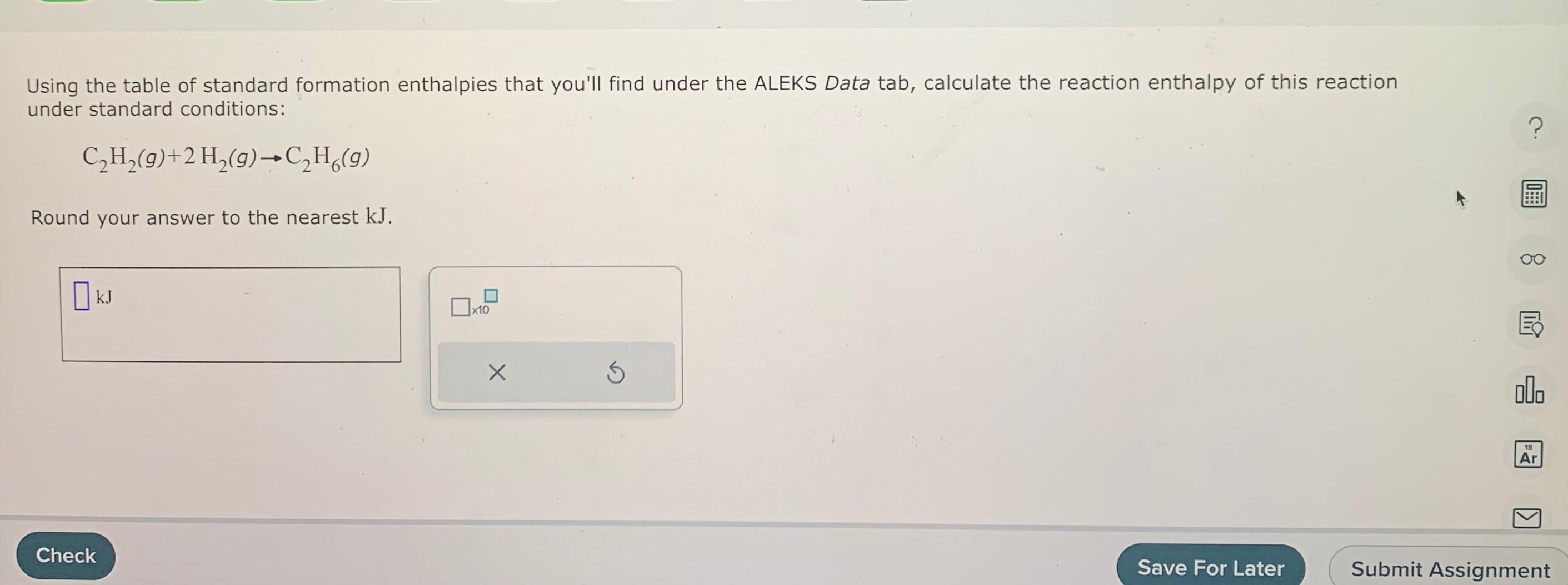
Solved Using the table of standard formation enthalpies that
The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. Determine the standard enthalpy of formation for ethylene glycol. What is the standard enthalpy of formation for ethanol c 2h 5oh?.
Solved Which of the following reactions is a standard
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. What is the standard enthalpy of formation for ethanol c 2h 5oh? Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. The standard enthalpy of formation, δh o f, is the.
Vidéo question Déterminer l’enthalpie standard de formation de l
1) the first thing to do is look up standard enthalpies of formation for the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. Balanced chemical equation for the formation of ethanol.
SOLVED 'Write a balanced chemical equation for the standard formation
Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of..
SOLVED6. Calculate the standard enthalpy change (AH) for the
Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. What is the standard enthalpy of formation for ethanol c 2h 5oh? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 1) the first thing to.
Solved Using the table of standard formation enthalpies that
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of..
PPT Heat of Formation PowerPoint Presentation, free download ID3890043
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Determine the standard enthalpy of formation for ethylene glycol. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 136 rows standard enthalpy.
What Is The Standard Enthalpy Of Formation For Ethanol C 2H 5Oh?
Determine the standard enthalpy of formation for ethylene glycol. 1) the first thing to do is look up standard enthalpies of formation for the. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1.
193 Rows In Chemistry And Thermodynamics, The Standard Enthalpy Of Formation Or Standard Heat Of Formation Of A Compound Is The Change Of.
The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.