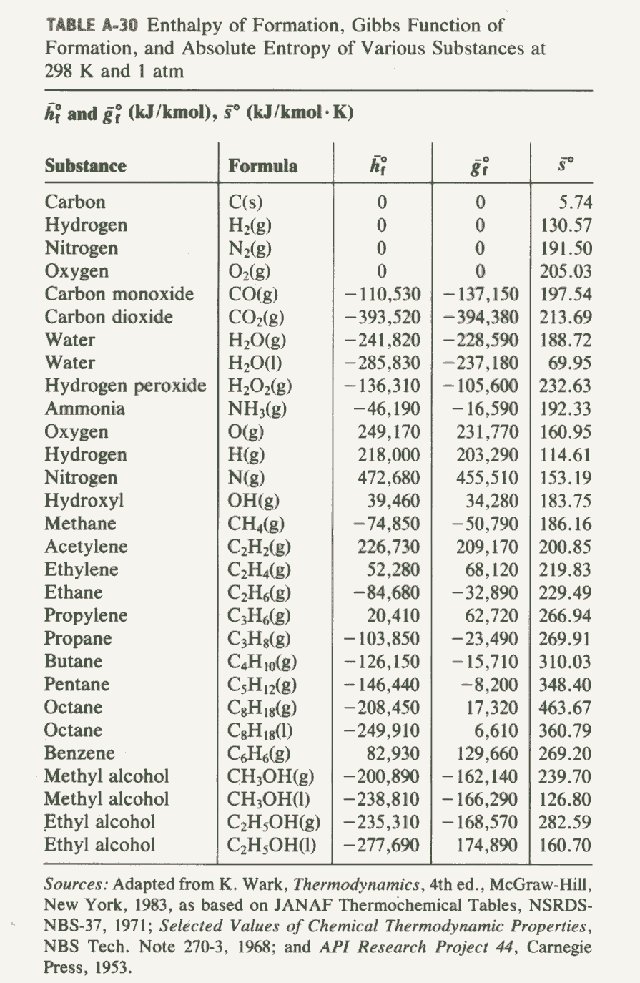
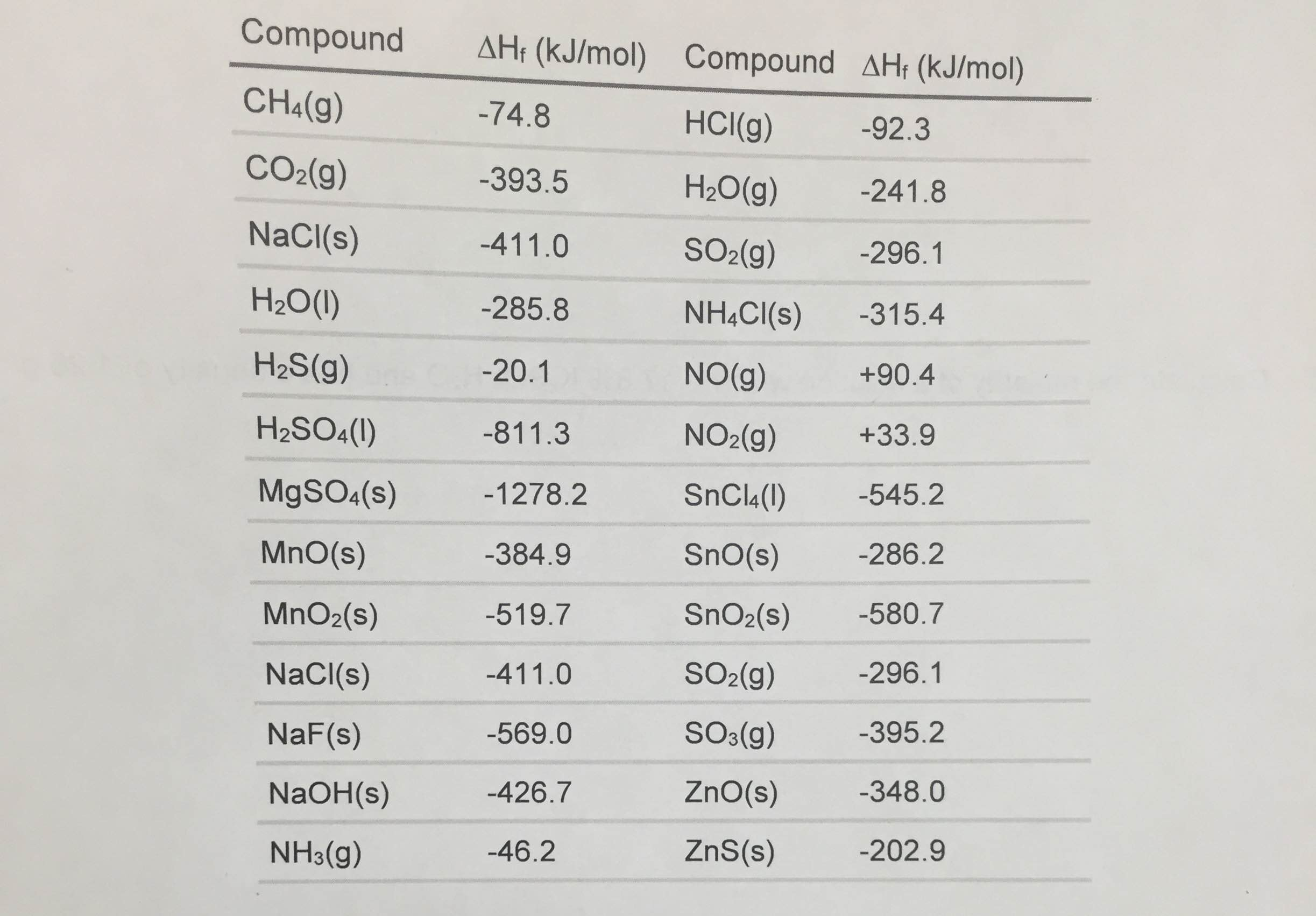
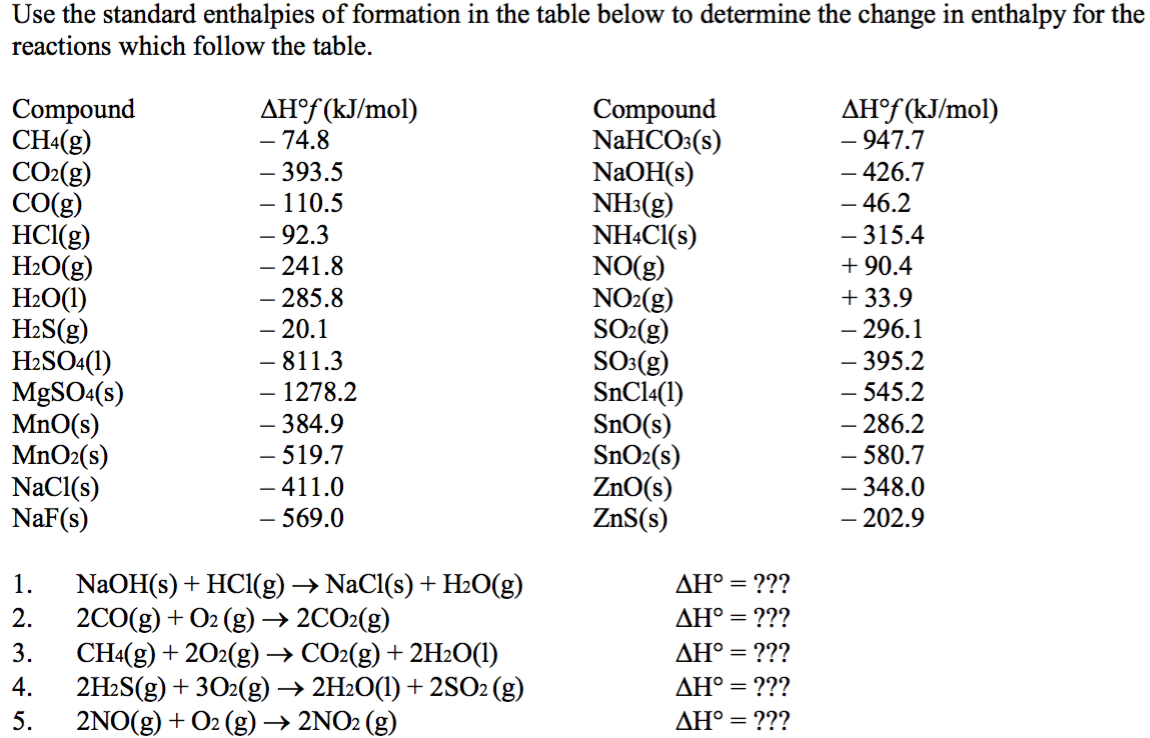
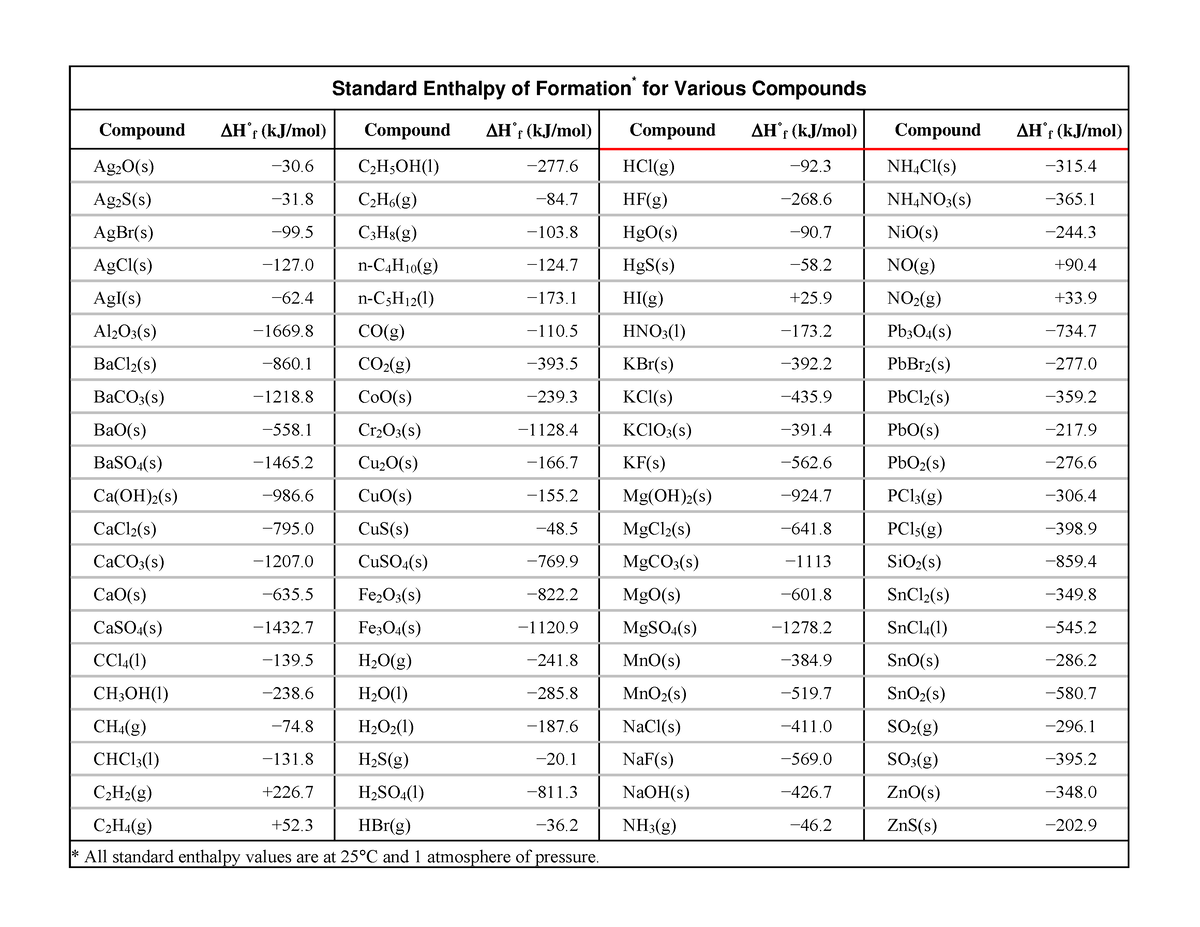
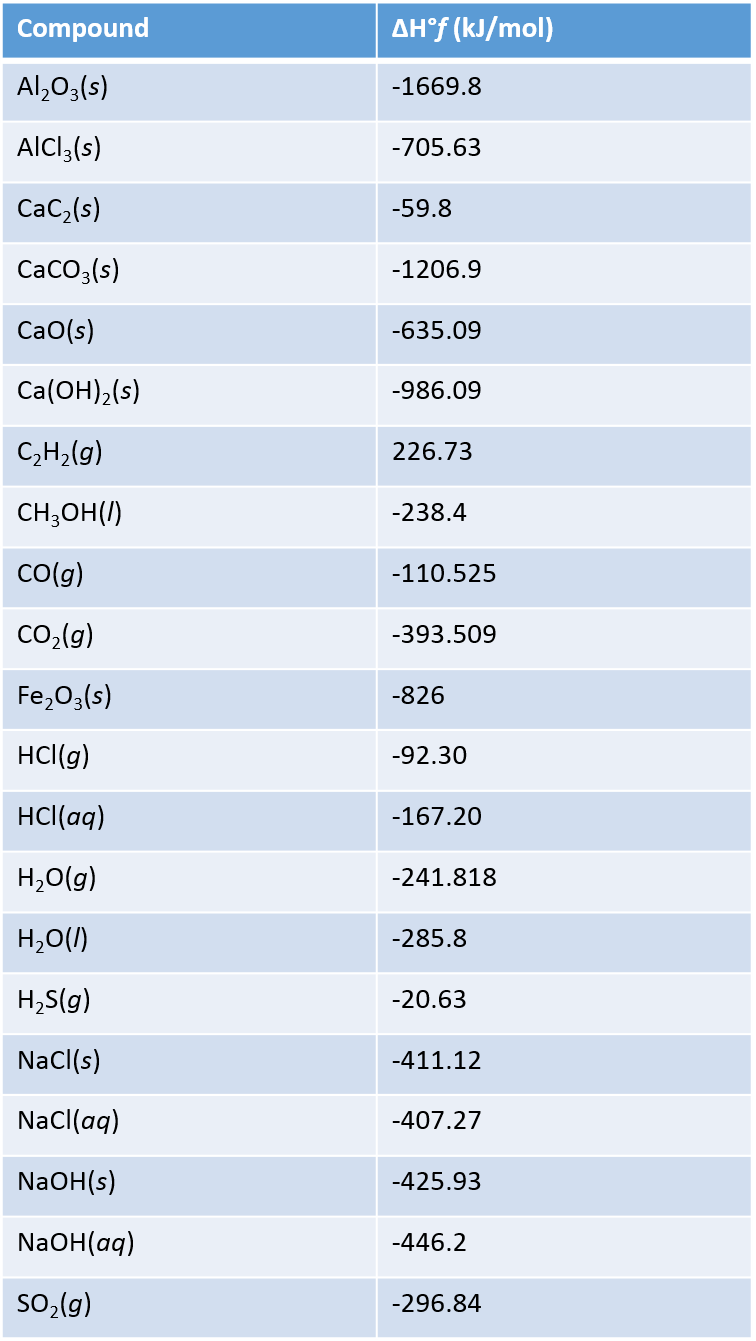
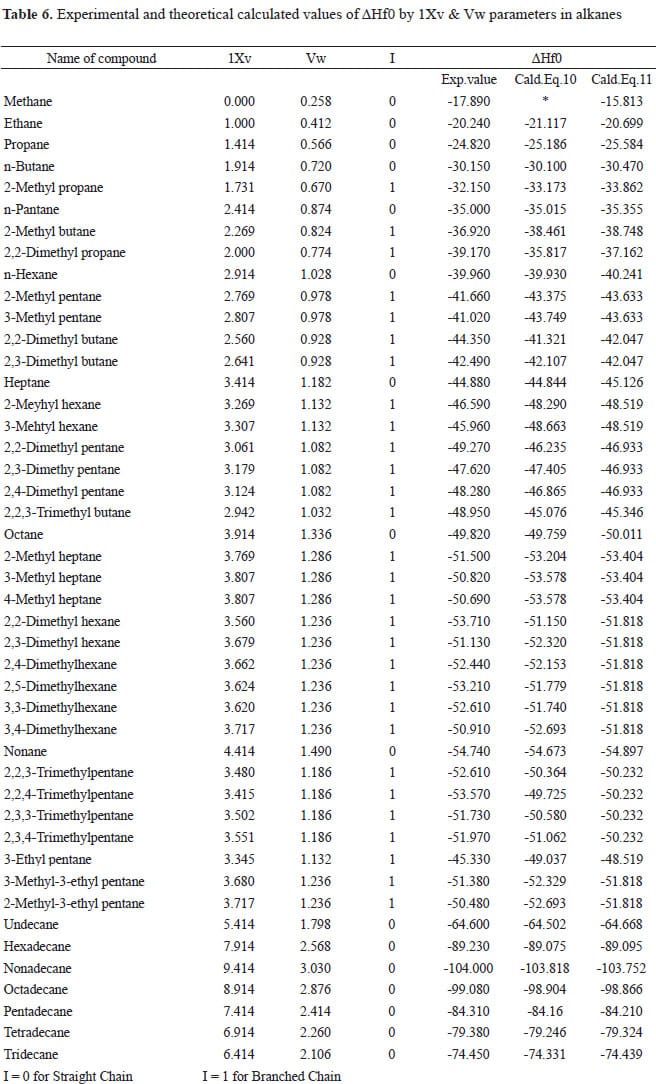
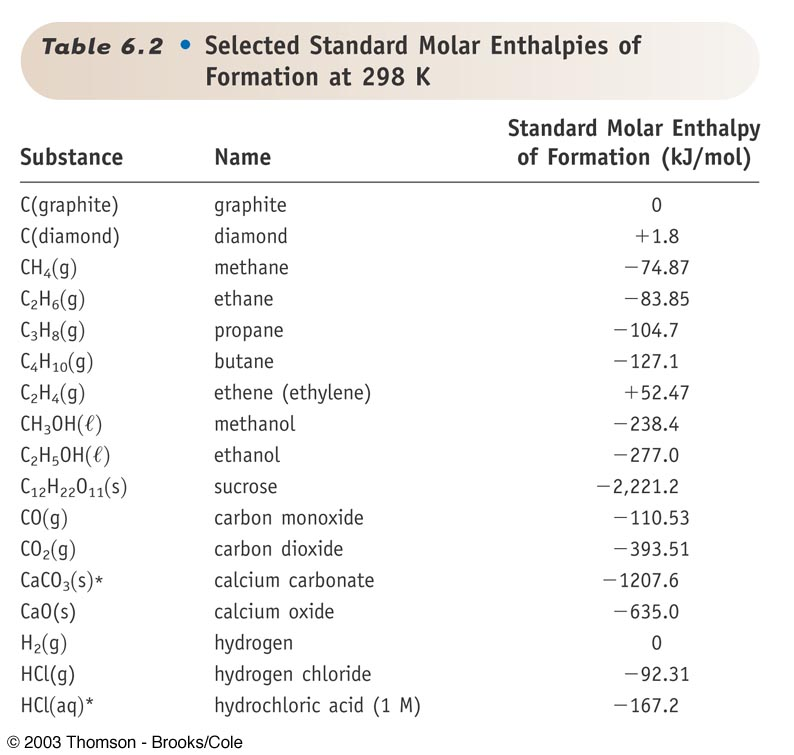
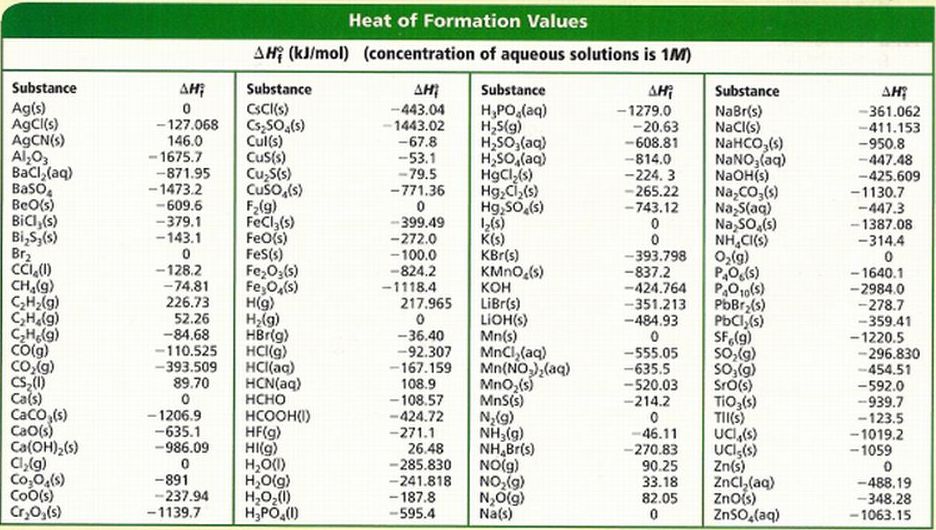
Table Of Standard Enthalpies Of Formation
Table Of Standard Enthalpies Of Formation - This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpy of formation* for various compounds compound δh˚ f (kj/mol) compound δh˚ f (kj/mol) compound δh˚ f (kj/mol). Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Table t1 is a more comprehensive table. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as.
Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Table t1 is a more comprehensive table. All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpy of formation* for various compounds compound δh˚ f (kj/mol) compound δh˚ f (kj/mol) compound δh˚ f (kj/mol).
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. Standard enthalpy of formation* for various compounds compound δh˚ f (kj/mol) compound δh˚ f (kj/mol) compound δh˚ f (kj/mol). This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as. Table t1 is a more comprehensive table. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.
Standard Enthalpies of Formation SchoolWorkHelper
All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard.
Solved Use a standard enthalpies of formation table to
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data.
Standard enthalpy of formation and standard free energy of formation of
Table t1 is a more comprehensive table. Standard enthalpy of formation* for various compounds compound δh˚ f (kj/mol) compound δh˚ f (kj/mol) compound δh˚ f (kj/mol). All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol).
Enthalpies of formation for stable and radical species used in work
Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Table t1 is a more comprehensive table. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 136 rows standard enthalpy change of formation (data table) these.
Solved Use the standard enthalpies of formation in the table
Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Table t1 is a more comprehensive table. The table below shows the standard enthalpy of.
Standard Enthalpy OF Formation C 2 H 2 (g) +226 H 2 SO 4 (l) −811
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Table t1 is a more comprehensive table. Standard enthalpy of formation* for various compounds compound δh˚.
Solved Using the table of standard enthalpies of formation
Table t1 is a more comprehensive table. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. Standand enthalpies of formation & standard entropies of common compounds.
Standard Enthalpies of Formation, ΔH°f SchoolWorkHelper
Standard enthalpy of formation* for various compounds compound δh˚ f (kj/mol) compound δh˚ f (kj/mol) compound δh˚ f (kj/mol). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Table t1 is a more comprehensive table. The table below shows the standard enthalpy of formation, the standard gibbs.
Solved Table 6.2. Selected Standard Molar Enthalpies of
Table t1 is a more comprehensive table. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. All values have units of kj/mol and physical conditions.
III Calculating Enthalpies STA Form IV Honors Chemistry
All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Table t1 is a more comprehensive table. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation.
Standard Enthalpy Of Formation* For Various Compounds Compound Δh˚ F (Kj/Mol) Compound Δh˚ F (Kj/Mol) Compound Δh˚ F (Kj/Mol).
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Table t1 is a more comprehensive table. All values have units of kj/mol and physical conditions of 298.15 k and 1 atm, referred to as.
This Table Lists The Standard Enthalpies (Δh°), The Free Energies (Δg°) Of Formation Of Compounds From Elements In Their Standard States, And The.
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.