What Is The Electron Pair Geometry For Xe In Xeo3
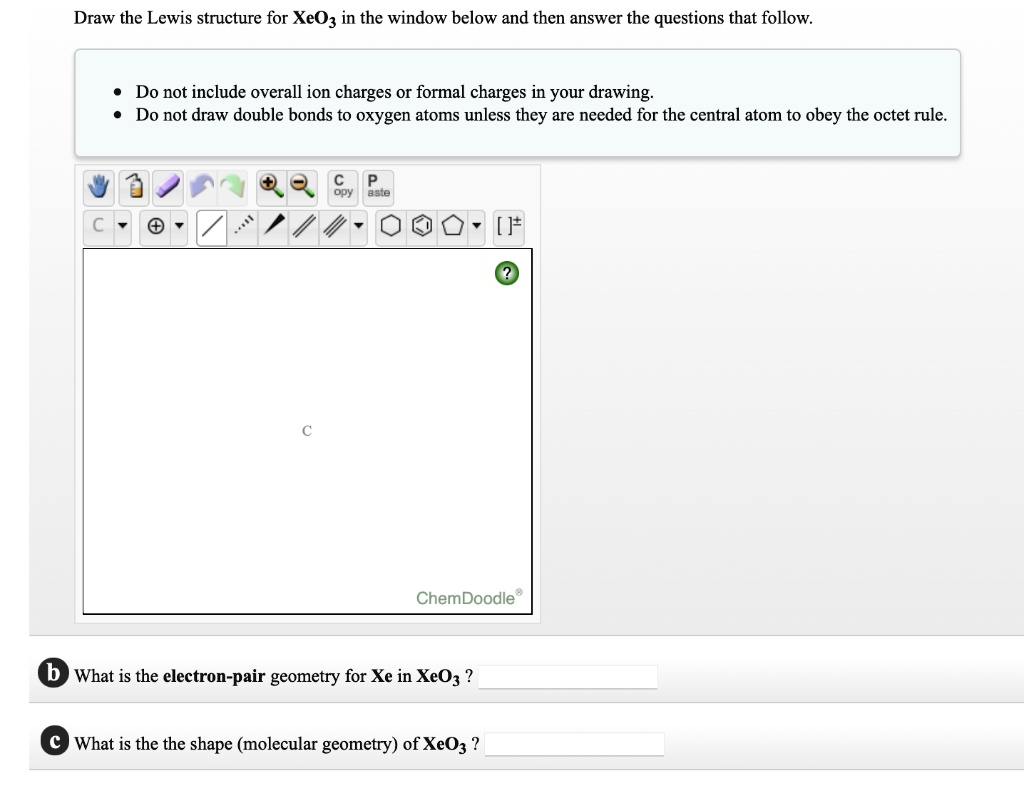
What Is The Electron Pair Geometry For Xe In Xeo3 - There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
SOLVED Draw the Lewis structure for XeOz in the window below and then
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
Shape of Molecules VSEPR Theory Affect Shape Of The Molecule
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
SOLVED Determine the electron geometry (eg), molecular geometry (mg
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
Spatial and electron pair geometry Molecular geometry, Teaching
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
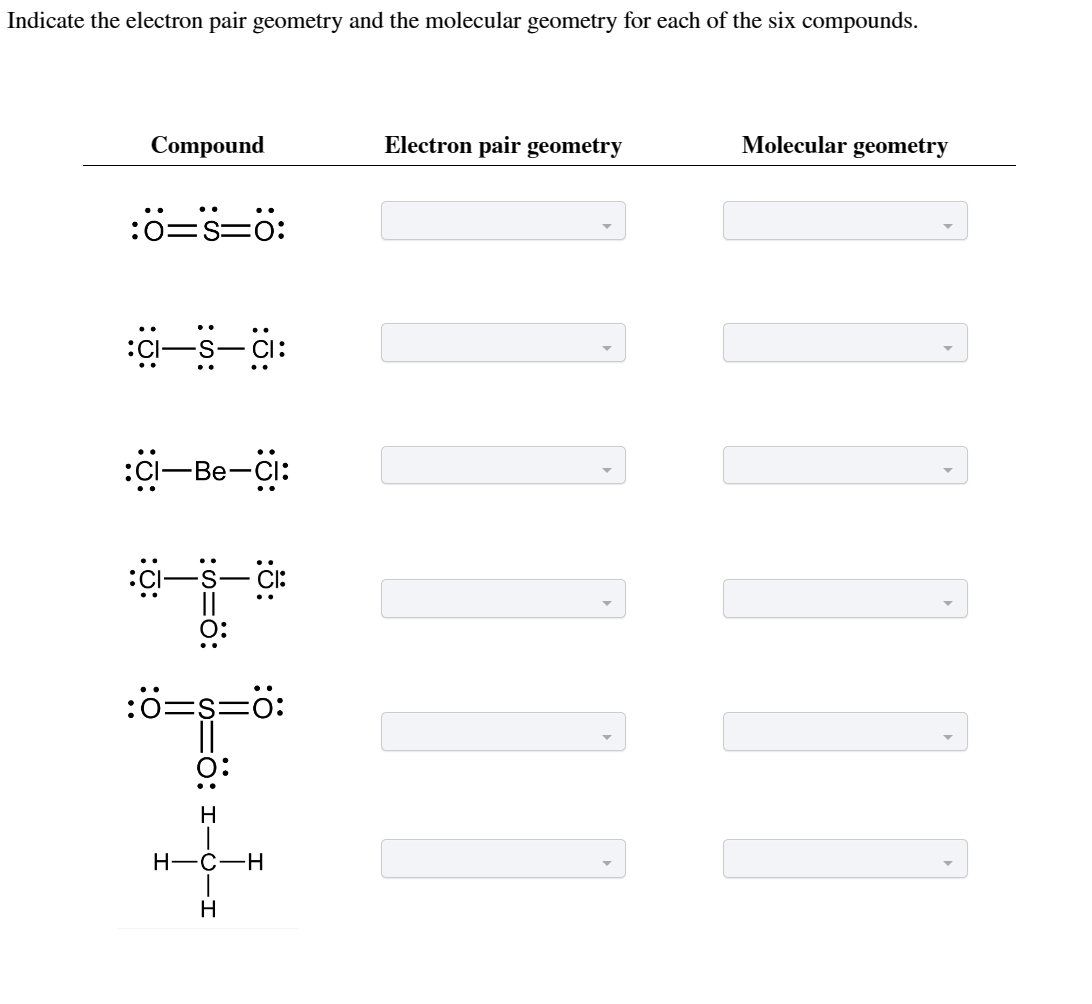
Solved (15)4. Predict the electron pair geometry and
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
Why the bond angle of XeO3 is 103 Chemistry Chemical Bonding and
The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no. There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.
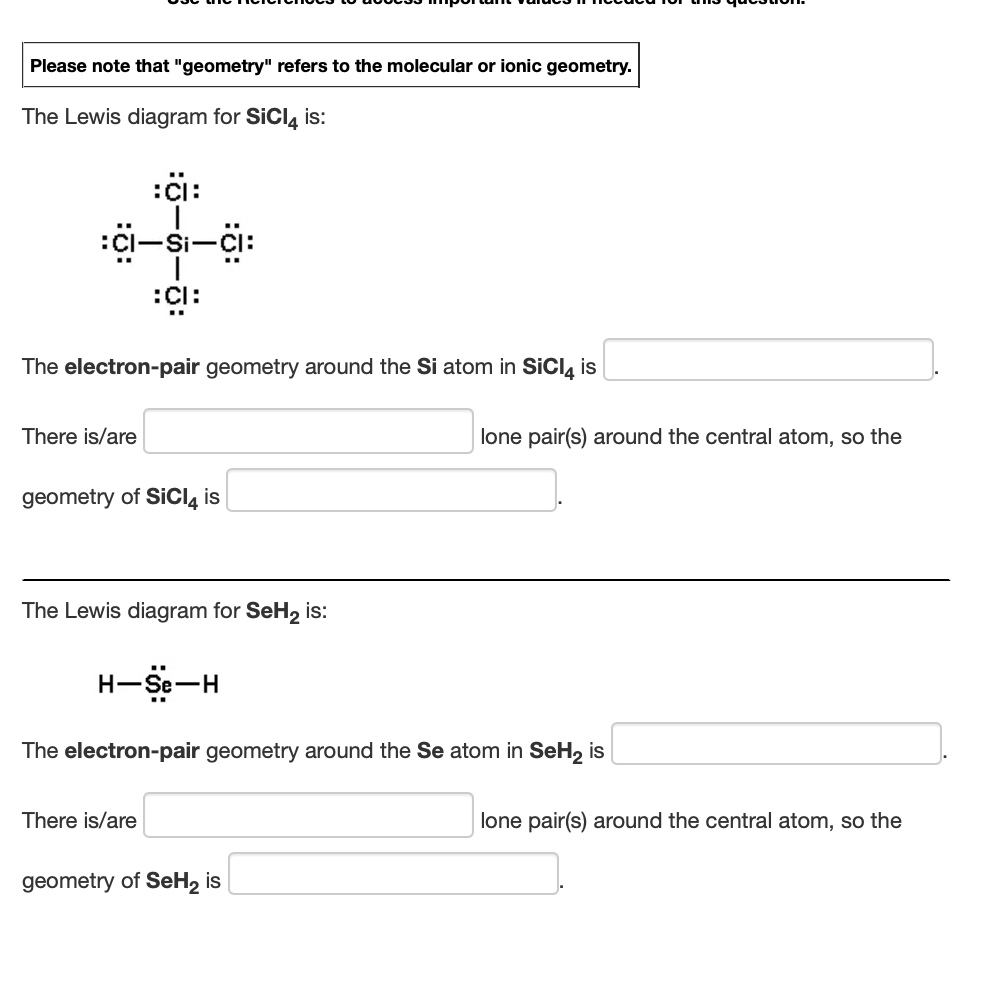
Solved The Lewis diagram for SiCl4 is The electronpair
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
Number of lone pairs of electrons on Xe atoms in XeF2, XeF4 and XeO3
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
[Solved] Predict the electronpair geometry and the molecular structure
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is. The structure of xenon trioxide comprises a central xenon atom around which 12 electrons or 6 electron pairs are present and no.
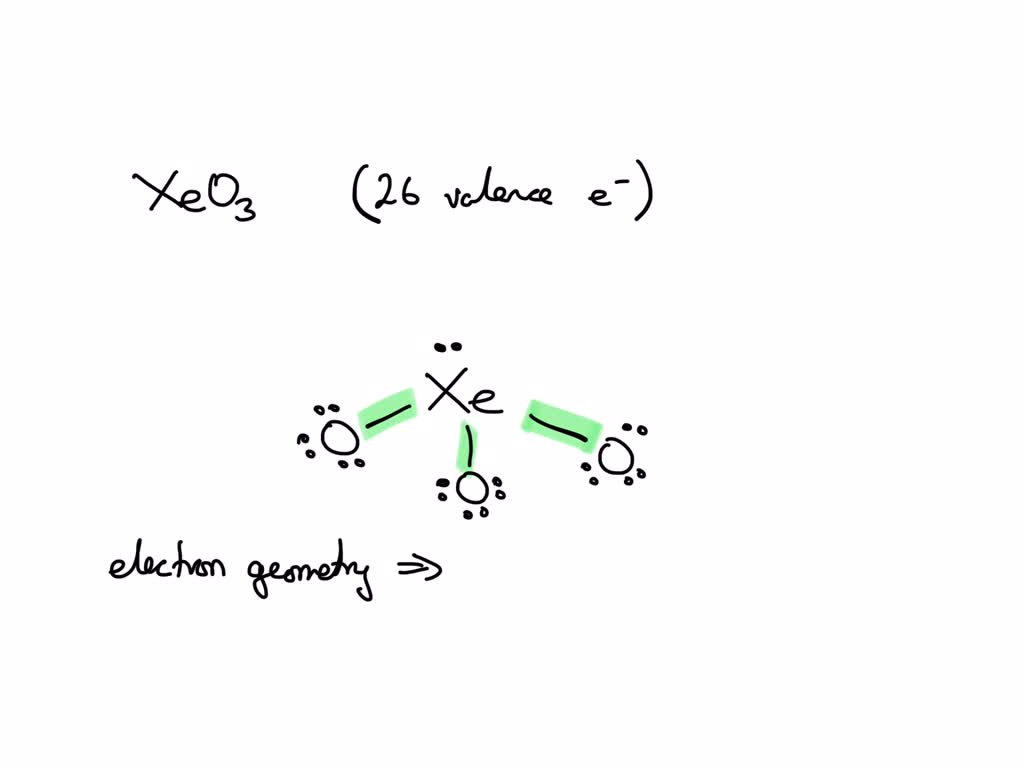
The Structure Of Xenon Trioxide Comprises A Central Xenon Atom Around Which 12 Electrons Or 6 Electron Pairs Are Present And No.
There are lone pair(s) around the central atom, so the molecular geometry (shape) of po(oh) 3 is.