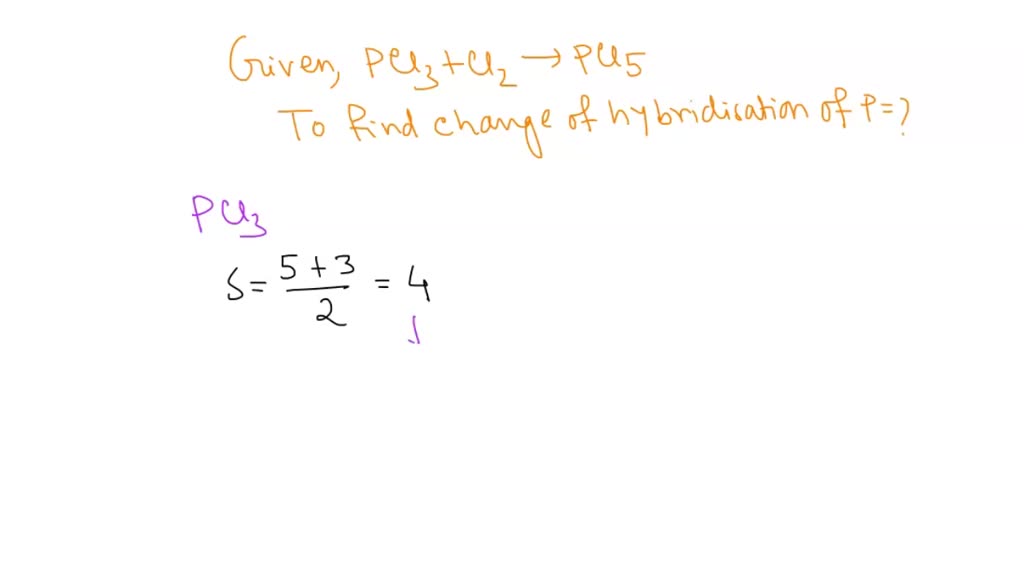What Is The Hybridization Of Phosphorus In Pcl3
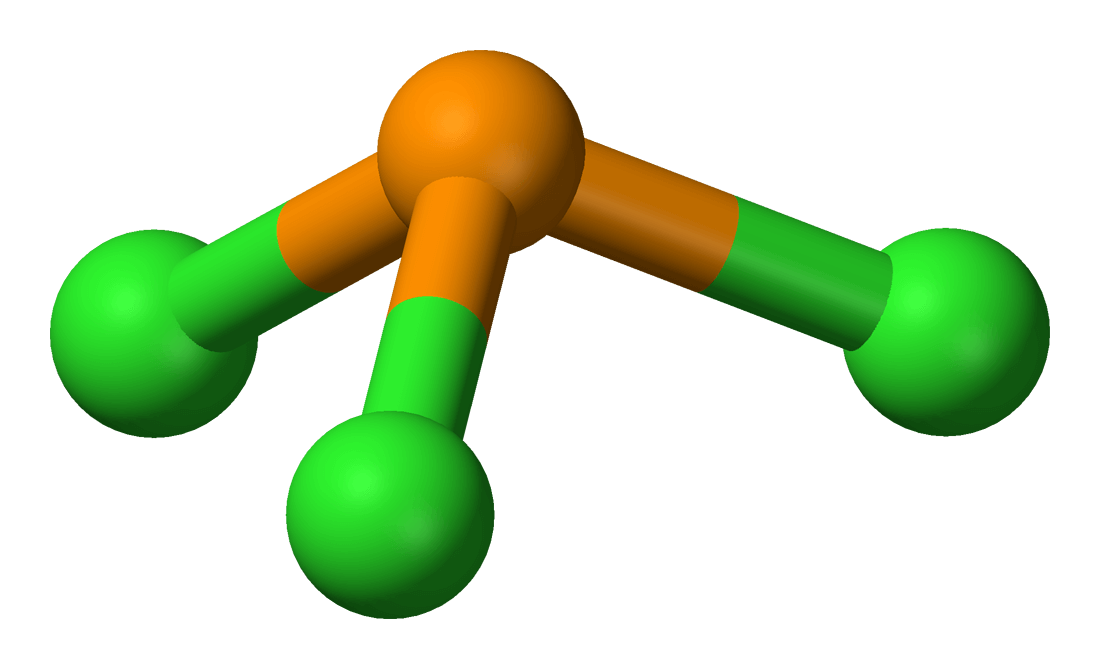
What Is The Hybridization Of Phosphorus In Pcl3 - Because the steric number of the phosphorous central atom is four. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. The phosphorus in pcl3 undergoes sp3 hybridization. Phosphorus in pcl 3 undergoes sp 3 hybridization. The hybridization of pcl 3 is sp 3. This is because it forms 4 bonds (3 sigma bonds. Here’s what this structure tells you:
To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. Here’s what this structure tells you: Phosphorus in pcl 3 undergoes sp 3 hybridization. This is because it forms 4 bonds (3 sigma bonds. Because the steric number of the phosphorous central atom is four. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. The phosphorus in pcl3 undergoes sp3 hybridization. The hybridization of pcl 3 is sp 3.
We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. The phosphorus in pcl3 undergoes sp3 hybridization. To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. The hybridization of pcl 3 is sp 3. Because the steric number of the phosphorous central atom is four. Here’s what this structure tells you: Phosphorus in pcl 3 undergoes sp 3 hybridization. This is because it forms 4 bonds (3 sigma bonds.
Hybridization of Orbitals Chemistry Topics Enseñanza de química
Phosphorus in pcl 3 undergoes sp 3 hybridization. Here’s what this structure tells you: This is because it forms 4 bonds (3 sigma bonds. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. The hybridization of pcl 3 is sp 3.
Lewis Electrondot Diagram For Pcl3
The hybridization of pcl 3 is sp 3. Here’s what this structure tells you: To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. Phosphorus in pcl 3 undergoes sp 3 hybridization. This is because it forms 4 bonds (3 sigma bonds.
PCL3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
The hybridization of pcl 3 is sp 3. Here’s what this structure tells you: The phosphorus in pcl3 undergoes sp3 hybridization. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry.
Хлорид фосфора (III) — свойства, получение и применение
Phosphorus in pcl 3 undergoes sp 3 hybridization. Because the steric number of the phosphorous central atom is four. The phosphorus in pcl3 undergoes sp3 hybridization. This is because it forms 4 bonds (3 sigma bonds. The hybridization of pcl 3 is sp 3.
Solved Determine the hybridization of phosphorus in each
Because the steric number of the phosphorous central atom is four. To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. The phosphorus in pcl3 undergoes sp3 hybridization. Phosphorus in pcl 3 undergoes sp 3 hybridization. The hybridization of pcl 3 is sp 3.
Solved Determine the hybridization of phosphorus in each
Because the steric number of the phosphorous central atom is four. The hybridization of pcl 3 is sp 3. Phosphorus in pcl 3 undergoes sp 3 hybridization. To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. The phosphorus in pcl3 undergoes sp3 hybridization.
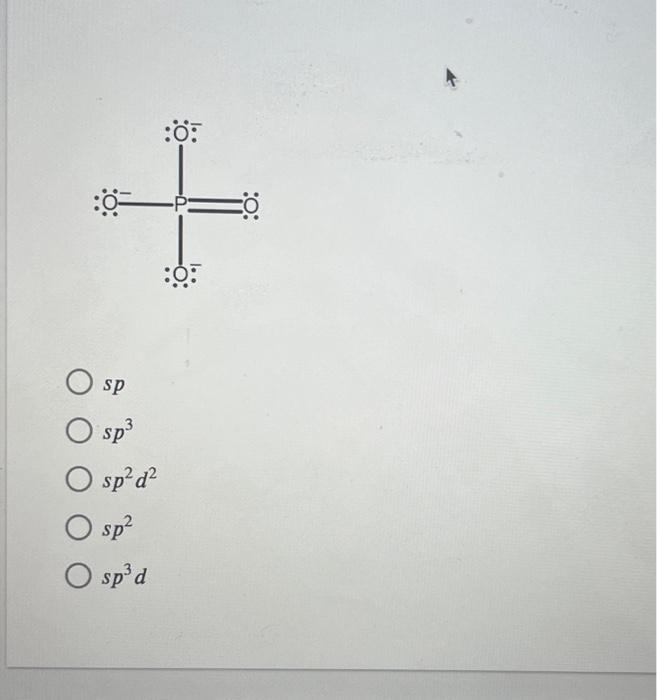
SOLVED Select the single best answer. In the following equation, what
We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. This is because it forms 4 bonds (3 sigma bonds. The hybridization of pcl 3 is sp 3. Here’s what this structure tells you: Phosphorus in pcl 3 undergoes sp 3 hybridization.
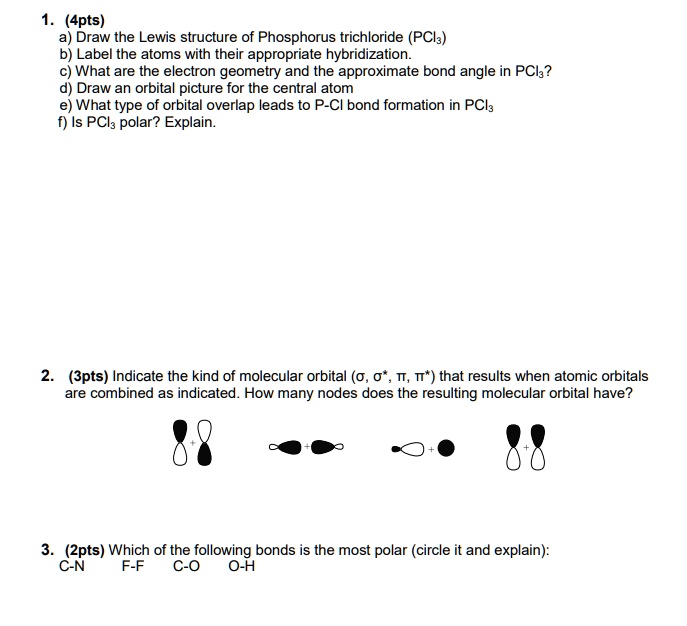
SOLVED Draw the Lewis structure of Phosphorus trichloride (PCl3
Phosphorus in pcl 3 undergoes sp 3 hybridization. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. This is because it forms 4 bonds (3 sigma bonds. Here’s what this structure tells you: To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at.
PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
Here’s what this structure tells you: Phosphorus in pcl 3 undergoes sp 3 hybridization. Because the steric number of the phosphorous central atom is four. The hybridization of pcl 3 is sp 3. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms.
Sp3d Orbitals
To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. The phosphorus in pcl3 undergoes sp3 hybridization. Here’s what this structure tells you: We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. This is because.
The Hybridization Of Pcl 3 Is Sp 3.
Because the steric number of the phosphorous central atom is four. To understand the hybridization scheme for pcl 3 (phosphorus trichloride), we need to look at its molecular geometry and the number of sigma. This is because it forms 4 bonds (3 sigma bonds. We can clearly see from the lewis diagram that in pcl3, phosphorus is forming three sigma bonds with 3 chlorine atoms.
The Phosphorus In Pcl3 Undergoes Sp3 Hybridization.
Phosphorus in pcl 3 undergoes sp 3 hybridization. Here’s what this structure tells you: