What Is The Hybridization Of The Central Atom In Sf4
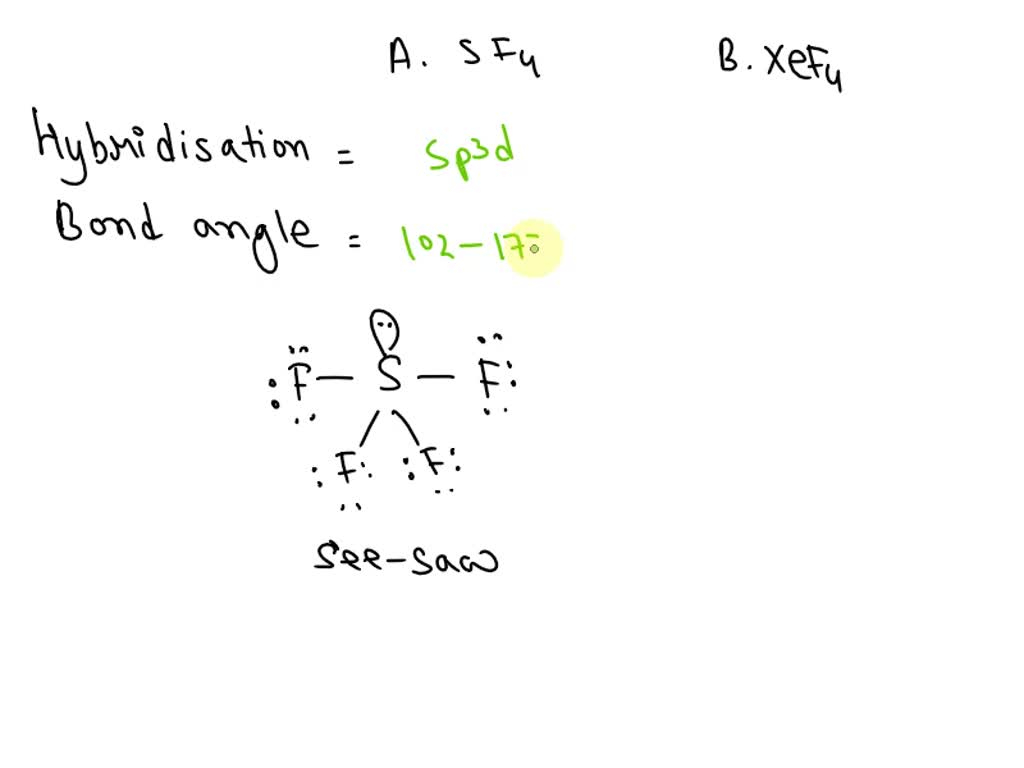
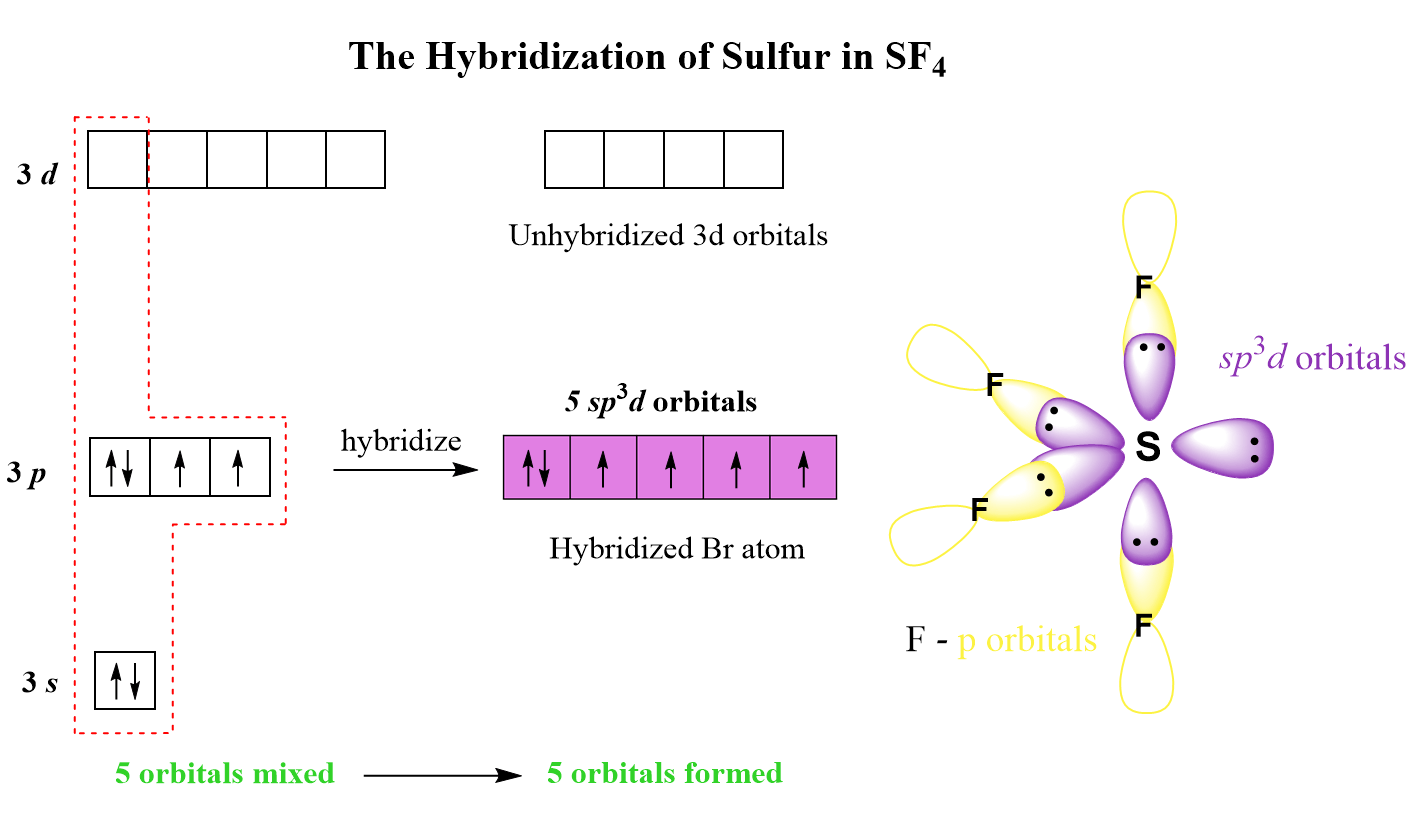
What Is The Hybridization Of The Central Atom In Sf4 - What is the hybridization of sf₄? The hybridization of sulfur in sf4 is sp3d. The bond angles in sf4 are approximately 120° and 90°. To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. The central atom is s. So, to explain in simple terms, its. In the bonding process, sulfur. The central atom in sf4 is sulfur (s). Sf₄ has only one lone pair and four sigma bonds of f. The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄.
The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°. Sf₄ has only one lone pair and four sigma bonds of f. What is the hybridization of sf₄? The central atom is s. To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. So, to explain in simple terms, its. The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄. The hybridization of sulfur in sf4 is sp3d. In the bonding process, sulfur.
The hybridization of sulfur in sf4 is sp3d. In the bonding process, sulfur. The bond angles in sf4 are approximately 120° and 90°. The central atom is s. So, to explain in simple terms, its. The central atom in sf4 is sulfur (s). The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄. To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. Sf₄ has only one lone pair and four sigma bonds of f. What is the hybridization of sf₄?
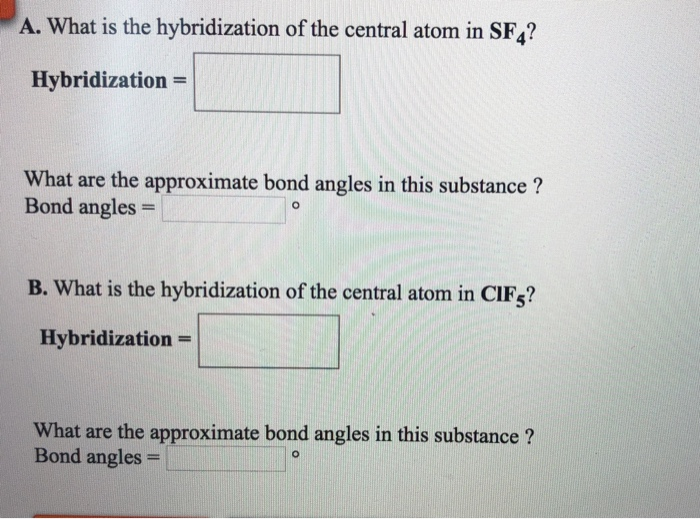
SOLVED A. What is the hybridization of the central atom in SF4
The central atom is s. What is the hybridization of sf₄? So, to explain in simple terms, its. In the bonding process, sulfur. The bond angles in sf4 are approximately 120° and 90°.
Sf4 Hybridization
To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. The central atom in sf4 is sulfur (s). So, to explain in simple terms, its. What is the hybridization of sf₄? The bond angles in sf4 are approximately 120° and 90°.
What is the hybridization of the central atom in SF4? ECHEMI
The bond angles in sf4 are approximately 120° and 90°. The central atom in sf4 is sulfur (s). What is the hybridization of sf₄? To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. The central atom is s.
Sf4 Hybridization
What is the hybridization of sf₄? In the bonding process, sulfur. Sf₄ has only one lone pair and four sigma bonds of f. The bond angles in sf4 are approximately 120° and 90°. The central atom in sf4 is sulfur (s).
Solved A. What is the hybridization of the central atom in
To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. In the bonding process, sulfur. Sf₄ has only one lone pair and four sigma bonds of f. The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄. The central atom is s.
What hybridization is generally utilized by the central atom in a
What is the hybridization of sf₄? So, to explain in simple terms, its. To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄. The bond angles in sf4 are approximately 120° and.
Tips and Tricks for Easily Determining Hybridization States of Atoms in
Sf₄ has only one lone pair and four sigma bonds of f. To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. The bond angles in sf4 are approximately 120° and 90°. The central atom in sf4 is sulfur (s). The hybridization of sulfur in sf4 is sp3d.
SF4 Geometry and Hybridization Chemistry Steps
Sf₄ has only one lone pair and four sigma bonds of f. The hybridization of sulfur in sf4 is sp3d. In the bonding process, sulfur. The central atom in sf4 is sulfur (s). So, to explain in simple terms, its.
Hybridization and Hybrid Orbitals ChemTalk
Sf₄ has only one lone pair and four sigma bonds of f. The central atom is s. The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°. So, to explain in simple terms, its.
[ANSWERED] A. What is the hybridization of the central atom in IF5
The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄. So, to explain in simple terms, its. Sf₄ has only one lone pair and four sigma bonds of f. The bond angles in sf4 are approximately 120° and 90°. The hybridization of sulfur in sf4 is sp3d.
The Hybridization Of Sulfur In Sf4 Is Sp3D.
To know the hybridization of the sf4 molecule, let us first look at the regions of electron density for the central. The hybridization of sulfur tetrafluoride is a significant characteristic, and its chemical formula is denoted as sf₄. So, to explain in simple terms, its. The bond angles in sf4 are approximately 120° and 90°.
The Central Atom In Sf4 Is Sulfur (S).
Sf₄ has only one lone pair and four sigma bonds of f. The central atom is s. In the bonding process, sulfur. What is the hybridization of sf₄?




![[ANSWERED] A. What is the hybridization of the central atom in IF5](https://media.kunduz.com/media/sug-question/raw/52262186-1659250506.6599846.jpeg?h=512)