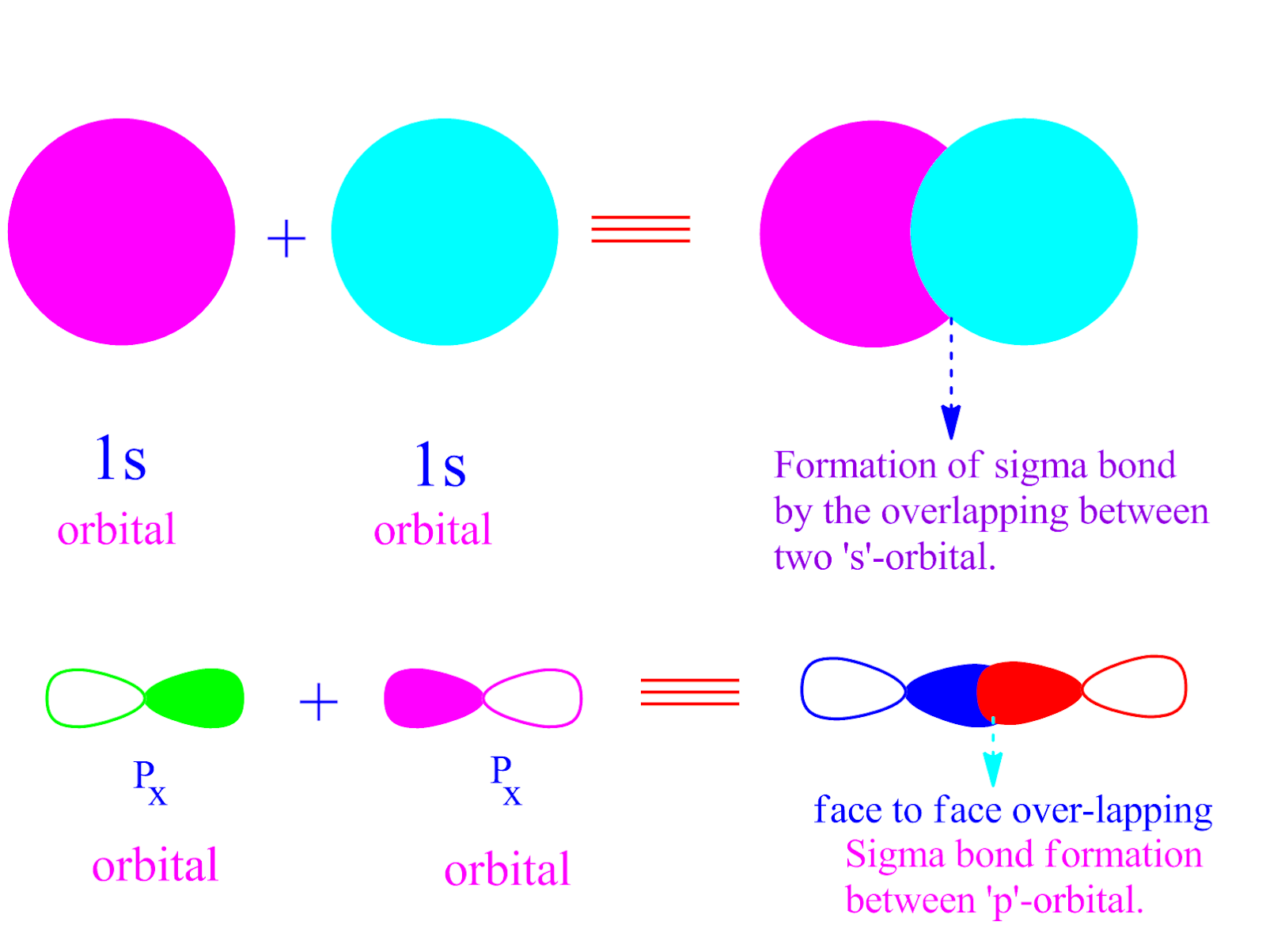
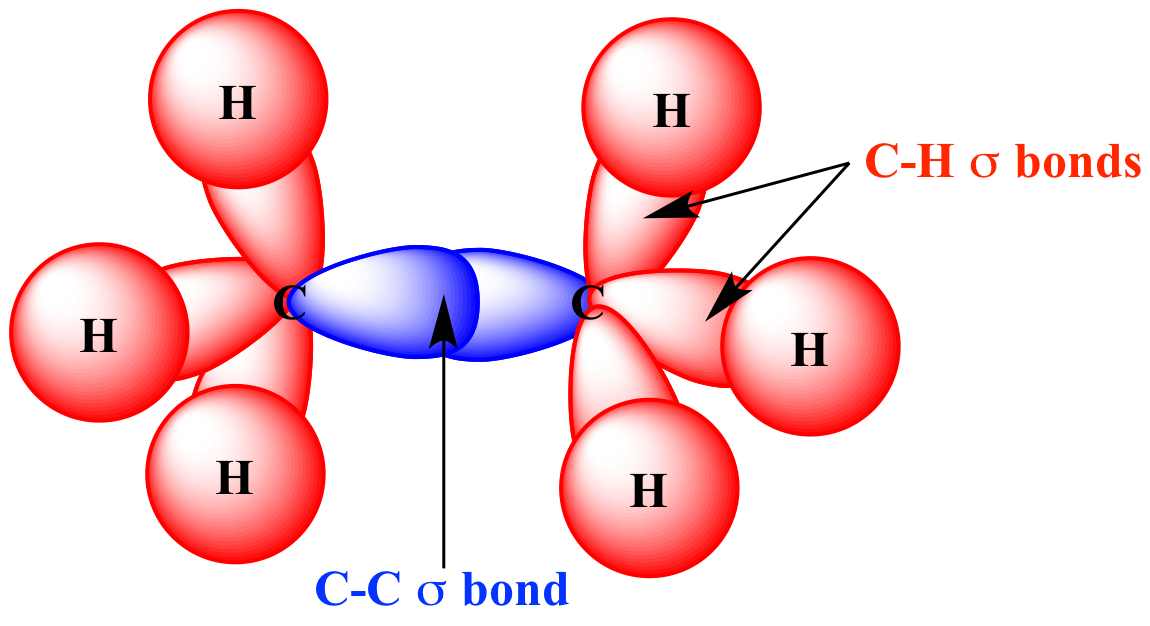
What Orbitals Form Sigma Bonds
What Orbitals Form Sigma Bonds - In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
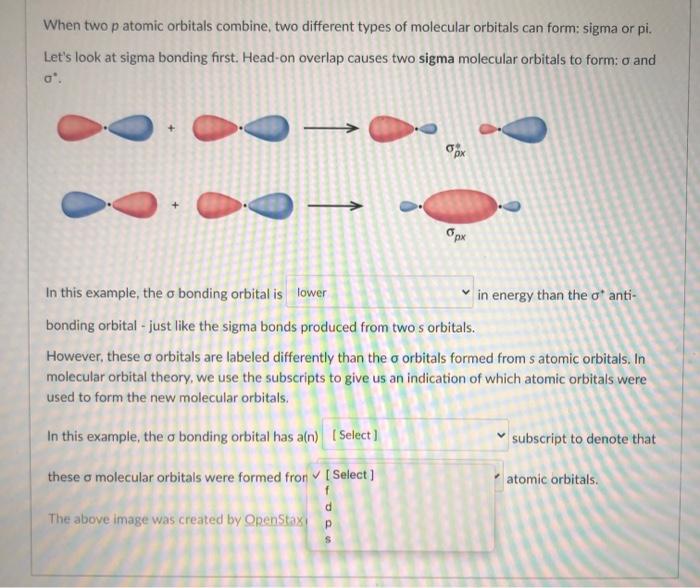
Solved When two p atomic orbitals combine, two different
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
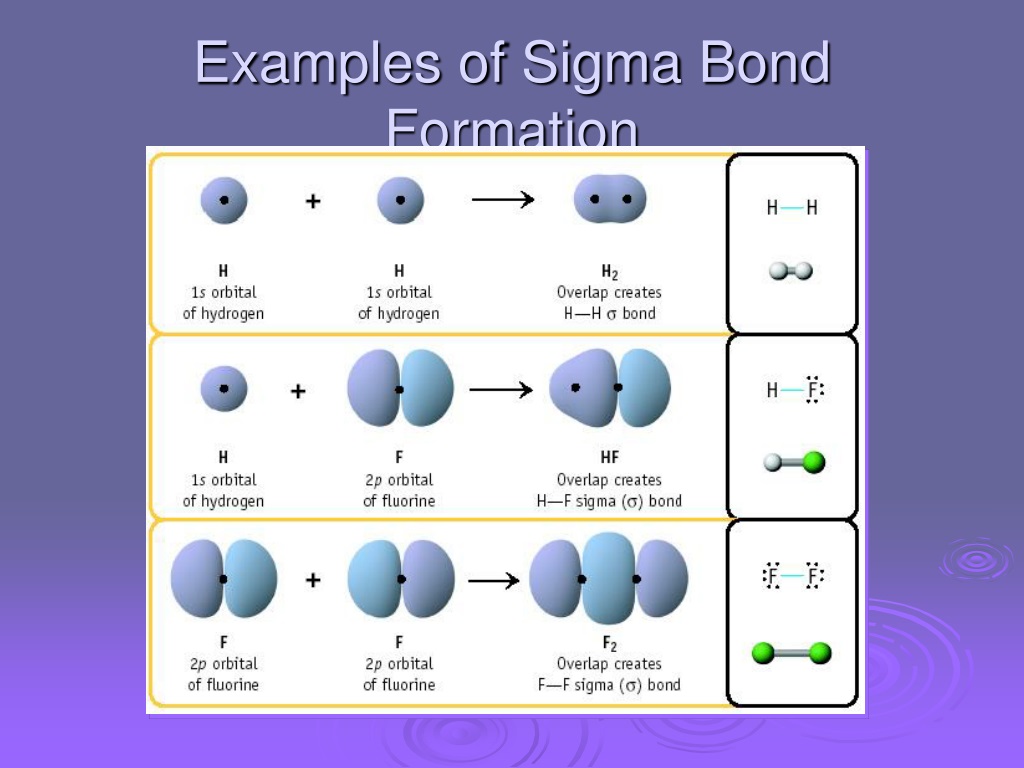
PPT Molecular Orbital Theory PowerPoint Presentation, free download
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
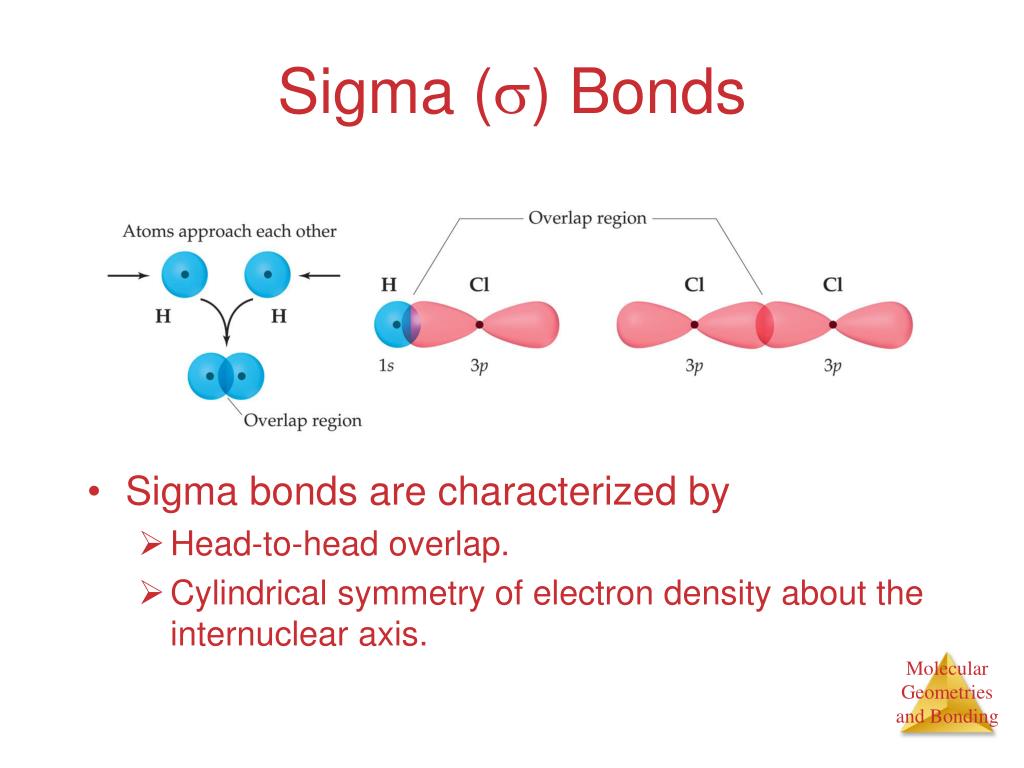
PPT Hybrid Orbitals PowerPoint Presentation, free download ID6008276
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
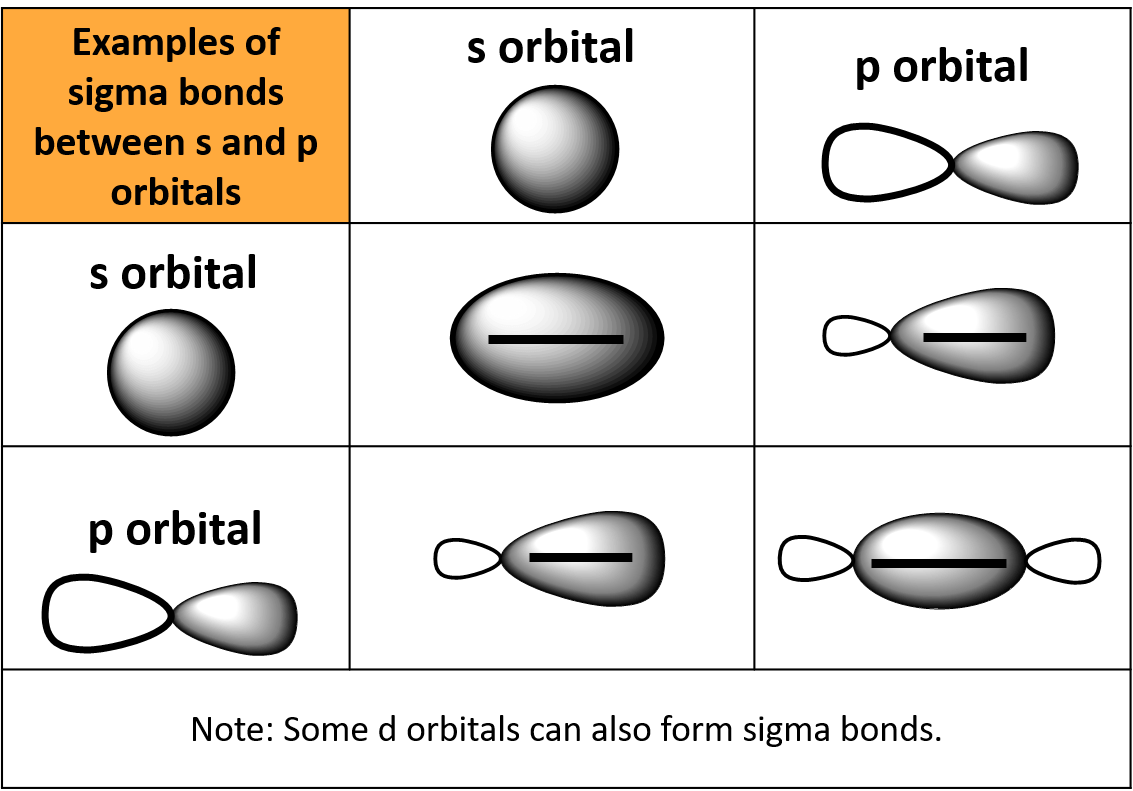
Sigma and Pi Bonds — Definition & Overview Expii
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
[Solved] Sketch the way orbitals overlap to form the sigma and pi bond
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
Sigma and Pi Bonds — Definition & Overview Expii
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
Illustrated Glossary of Organic Chemistry Sigma bond (σ bond)
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.
Sigma and Pi Bonds Brilliant Math & Science Wiki
In transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry (and energy) to form.