Which Group Tends To Form 1 Ions
Which Group Tends To Form 1 Ions - Which group tends to not form ions or react? Which group on the periodic table tends to form 1+ ions? Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. They have 1 valance electron to lose to become a noble gas. Group 2 metals, the alkaline. Whats the opposite of electron. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
Group 2 metals, the alkaline. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Whats the opposite of electron. Which group tends to not form ions or react? Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
They have 1 valance electron to lose to become a noble gas. Whats the opposite of electron. Group 2 metals, the alkaline. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form 1+ ions? Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
Molecular and Ionic Compounds CHEM 1305 General Chemistry I—Lecture
Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group tends to not form ions or react? Group 2 metals, the alkaline. Whats the opposite of electron. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when.
What Is The Charge Of Group 13 On Periodic Table
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Whats the opposite of electron. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 2 metals, the alkaline. Which group on the periodic table tends to.
SOLVED ATOMIC RADIUS What trend in atomic radius do you see as you go
They have 1 valance electron to lose to become a noble gas. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Which group tends to not form ions or react? Group 2 metals, the alkaline. Whats the opposite of electron.
Question Video Connecting the Group Number with the Subscript in an
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Which group on the periodic table tends to form 1+ ions? Whats the opposite of electron. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 2.
Ionic Compound Nomenclature Presentation Chemistry
Which group on the periodic table tends to form 1+ ions? Which group tends to not form ions or react? Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions.
O Ion 19 39k+1 Possui
Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline. They have 1 valance electron to lose to become a noble gas. Whats the opposite of electron.
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest
Group 2 metals, the alkaline. Which group on the periodic table tends to form 1+ ions? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Which group tends to not form ions or react? They have 1 valance electron to lose to become a noble gas.
Solved Sort the following statements based on whether demand
Whats the opposite of electron. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. They have 1 valance electron to lose to become a noble gas. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline.
Metals and nonmetals The Basics Reactivity Reactions with
Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Whats the opposite of electron. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Group 2 metals, the.
Is Oxygen a Positive or Negative Ion? Infrared for Health
Group 2 metals, the alkaline. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Whats the opposite of.
Group 1 Metals, The Alkali Metals, Have The 1 Valence Electron, And Thus Form M^+ Ions When Oxidized.
Whats the opposite of electron. They have 1 valance electron to lose to become a noble gas. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline.
Study With Quizlet And Memorize Flashcards Containing Terms Like Which Group Tends To Form 1+ Ions?, Which Group Tends To Form 2+ Ions?, Which.
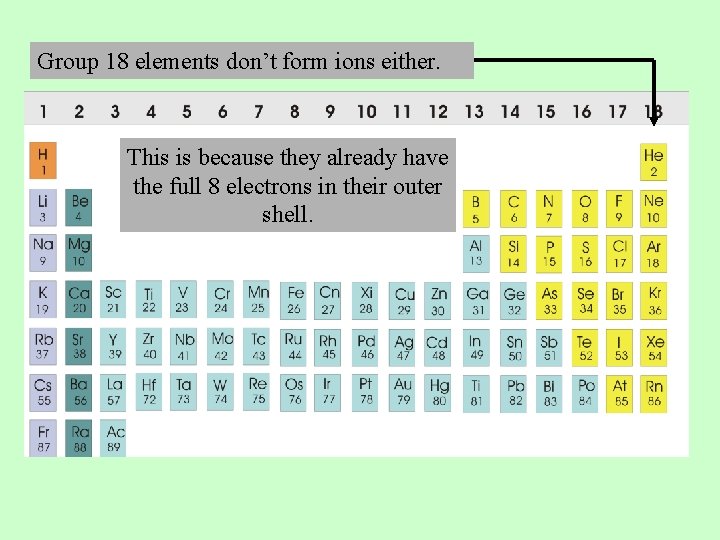
Which group tends to not form ions or react?




.PNG)